The molecule CH4 has a total of how many valence electrons
What is 8?
Resonance, isomers, or identical 
What are isomers?
Formal charge for C in CH4
What is 0?
Molecular geometry for a water molecule
What is bent?
Methane: Polar covalent, nonpolar covalent, ionic, or metallic?
What is nonpolar covalent?
When an atom of certain elements can have greater than an octet of electrons in its Lewis structure
What is hypervalent?
Resonance hybrid of what molecule (the name pls)
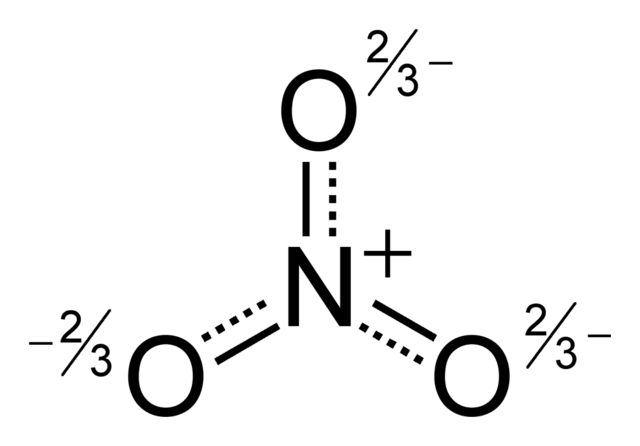
What is nitrate?
Formal charge for C in carbon dioxide
What is 0?
Molecular geometry for an ammonia molecule
What is trigonal pyramidal?
magnesium hydroxide: Polar covalent, nonpolar covalent, ionic, or metallic?
What is ionic?
A molecule of nitrogen has how many valence electrons
What is 28?
The ozone molecule: does it have a resonance structure, isomer, both, or neither
What is resonance?
Formal charge for C in carbon monoxide
What is -1?
Molecular geometry for an ammonium molecule
What is tetrahedral?
Name the element that can be hypovalent or also form an octet
What is boron?
C2H3O2 1- has how many valence electrons
What is 24?
Which is are isomers of hexane?

b,c,d,e
Formal charge for S in thiocyanate
What is -1?
Central atom bond angle for boron tribromide
What is 120 degrees?
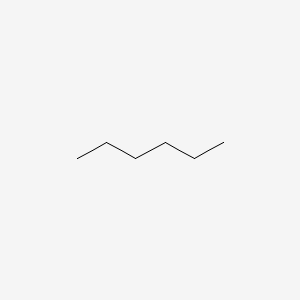
Name the molecule:
What is hexane?
Phthalate has how many valence electrons
What is 62?
On the board, draw two Lewis Structures:
Pentane & an isomer
Mr. C will check
Formal charge for the central O in ozone
What is +1?
Central atom bond angle for sulfur dioxide
What is 118?
Come to the board ad write the balanced reaction in terms of Lewis structures (be sure to show lone pairs): decomposition of water
O2 has a double bond