This is a positively charged particle in the nucleus of the atom.
What is a proton?
True or false: heterogenous mixtures can be a mixture of solids, liquids, and/or gases.
True.
Mixtures can be any combination of all states of matter: solids, liquids, and gases.
What is Group 15 (new Periodic Table) or Group VA?
Isotopes
What is the electron structure or electron notation of Nitrogen?
2,5
This is an uncharged particle in the nucleus of the atom.
What is the smallest unit of an element that maintains all the properties of that element?
Atoms
What makes up the majority of the periodic table?
What are metals?
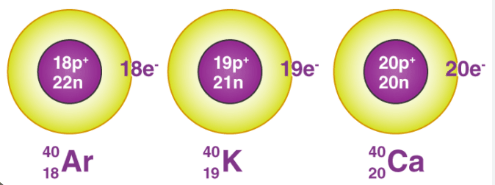
Isobar
What element is this?

Cobalt
The number of protons for the element Calcium (Ca)
Is a heterogeneous mixture chemically bonded? Elaborate.
No. Heterogeneous mixtures are combined but not chemically like compounds.
This represents the identity of an element and does not change for that element.
What are protons?
calcium ions and chloride ions
isoelectronic
What is the electron notation of the element below?

2, 8, 3
Where are protons located in an atom?
nucleus
What is another term we can use for homogeneous mixtures that contains a solute and solvent?
Solution
In this category, a mystery element Z is a non-lustrous solid and a poor conductor of electricity.
What are nonmetals?

Isotones
What are nucleons?
number of protons and neutrons
How many neutrons does the following element have?

What is 61 neutrons?
Which of these statements are true about compound?
A. Compounds can be found in the periodic table.
B. Compounds are pure substances.
C. Compounds are made up of two or more pure substances.
D. Compounds can be separated by physical means.
B and C
Compounds are pure substances and are made up of two or more elements.
*Elements can be found in the periodic table.
*Compounds can only be separated by chemical means.
The seven diatomic elements found in nature (meaning they are found bonded to themselves).
What are Br, I, N, Cl, O, H, and F?
Hg-196 and Hg-199
How many neutrons are in tellurium?
76 neutrons