What does the “IV” mean in tin (IV) iodide?
charge of tin (cation) is +4
How many electrons are shared in a triple covalent bond?
6
Why do ionic compounds transfer electrons?
To get 8 valence electrons and be stable.
How many valence electrons are present in an atom of iron?
2
What type of bond forms when electrons are shared equally between two atoms?
nonpolar covalent bond
What is the chemical formula for phosphorus pentachloride?
PCl5
What does the prefix “hepta” mean
7
Which types of atoms form covalent bonds?
Two nonmetals
What is an anion?
A negatively charged ion that has gained electrons.
List an element that forms a +2 ion.
All transition and inner transition metals, as well as group 2 alkaline earth metals.
What is the correct name for Mg2C?
Magnesium carbide
What is the VSEPR shape for methane (CH4)?
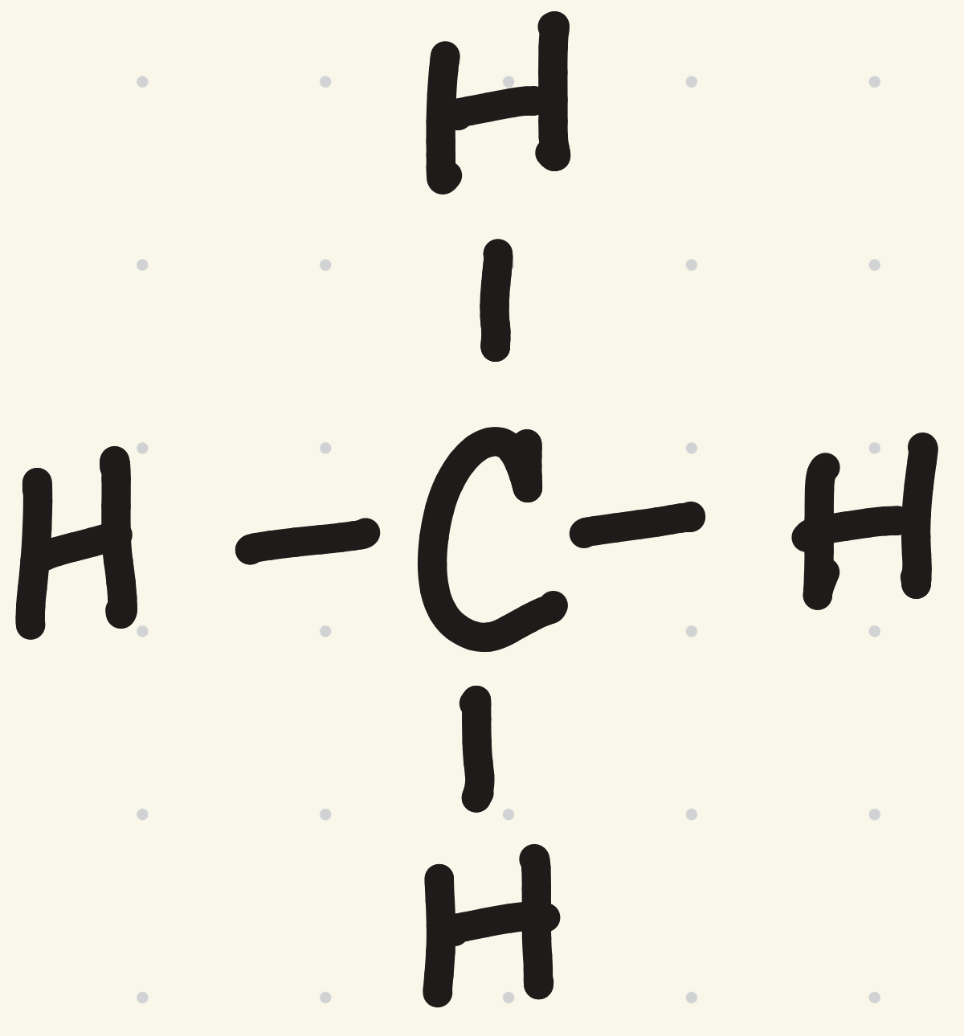
tetrahedral
List a property of covalent compounds
Low melting and boiling points
Poor conductors of heat and electricity
Are liquids and gases at room temperature
What charge will phosphorus have when it becomes an ion?
-3
Draw the Lewis Dot diagram for a neutral atom of Germanium

What is the correct formula for chromium (III) sulfate?
Cr2(SO4)3
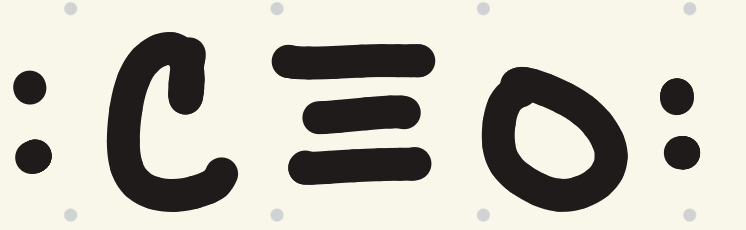
Draw the Lewis structure for carbon monoxide
Atoms with very different electronegativities are expected to form ______ bonds
ionic bonds
How does selenium obtain a full octet when forming ionic bonds? What is its charge?
gains 2 electrons, has a -2 charge
What is the effect of lone pairs on molecule polarity?
It makes them polar
What ionic compound forms when nitrate and aluminum react?
Al(NO3)3
Which VSEPR shapes are nonpolar?
Linear, trigonal planar, tetrahedral
What is the name for the type of bonding in metallic compounds?
Electron sea model (electrons flow between atoms)
What is the charge on iron in Fe(NO3)3?
+3
What are the 7 diatomic atoms?
H, N, F, O, I, Cl, Br (Have no fear of ice cold beverages)