What is an electric balance good for?
Good for measuring mass
what is the name of this element Mn?
Manganese
What is the definition of atomic mass?
Mass of a single atom of a chemical element
What is the rule of zero charge?
When an ionic compound forms the total charge on the atoms add up to zero
How many bonds does each element have in the HONC 1234 rule?
H is 1 bond
O is 2 bonds
N is 3 bonds
C is 4 bonds
What is something you use to cover your eyes when around chemicals?
goggles
what does the law of conservation of matter state?
matter can neither be created nor destroyed
What are the 3 main subatomic particles?
Protons, neutrons, electrons
How many valence electrons does argon have?
8 valence electrons
True or False:
Neon does not follow the octet rule
False it does 8 valence electrons
What is something that has matter?
has to have mass and matter for example air
what is this element La?
Lanthanum
How many isotopes does strontium Sr have?
4
what kind of ion is Sodium Hydroxide (NaOH)?
polyatomic ion
give an example of ionic and covalent compounds?
example of an ionic compound: NaCl
example of a covalent compound: CH4
how do you calculate density?
D= M/V
What is the compound of salt?
Sodium Chloride NaCl
what is the average atomic mass of titanium?
47.867u
What's the name of this polyatomic ion (NH4NO3) ?
ammonium nitrate
What is the molecular formula for Ribose?
C5H10O5
what is the volume of pure 31.1 g of gold?
the volume is 1.61 cubic centimeters
what is the periodic trend of non-metallic?
Non-Metallic: left to right, Bottom to top
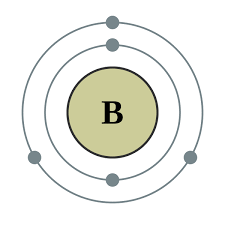
what is the atomic model of Boron?
What element is this if the flame color blue?
copper
Draw the structural formula Menthol C10H18O