The element hydrogen is classified as a:
Pure Substance
A property that does NOT depend on the amount of matter is an _________________ property
intensive
What is the formula for density?
mass divided by volume
 _____________is the state of matter with an indefinite shape but a fixed volume, and takes the shape of its container.
_____________is the state of matter with an indefinite shape but a fixed volume, and takes the shape of its container.
Liquid
Which classification of matter is table salt, NaCl?
- Element
- Compound
- Het Mixture
- Hom Mixture
Compound
____________ are combinations of two or more pure substances
Mixture
 This is the GHS symbol for flammable. Flammable is what type of property?
This is the GHS symbol for flammable. Flammable is what type of property?
Chemical
If mass stays the same, but volume DECREASES, what happens to the density?
Density gets bigger
 _______is the state of matter with the most space between particles, has no definite shape or fixed volume, and takes the shape of its container.
_______is the state of matter with the most space between particles, has no definite shape or fixed volume, and takes the shape of its container.
Gas
Which classification of matter is Carbon, C?
- Element
- Compound
- Het Mixture
- Hom Mixture
The compound NaCl, table salt, is classifed as a:
Pure substance
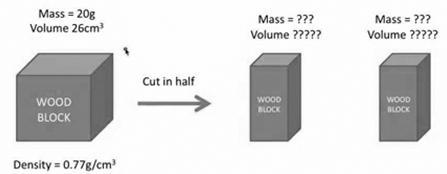
What is the mass of block A?
10 g
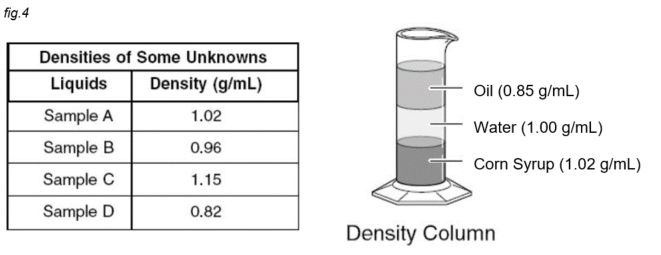 Which sample would float on top of water, but not the oil?
Which sample would float on top of water, but not the oil?
Sample B, because its density of 0.96 is between 0.85 and 1.00
A piece of metal is dropped into a clear liquid. Bubbles suddenly appear. When a lit match is put into the gas, a loud “POP” occurs. This is evidence of a __________.
chemical change
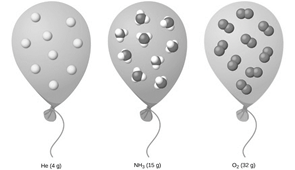
Why do the gases in the picture represent matter?
The gases have mass and take up space
Iced bubbly soda is a ______________ mixture 
heterogeneous mixture
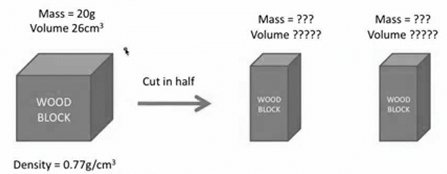 What is the volume of block A?
What is the volume of block A?
13cm3
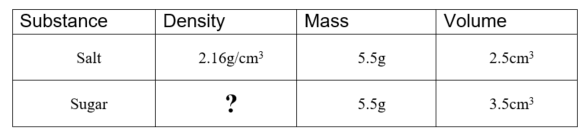 What is the density of sugar? Round your answer to two decimal places.
What is the density of sugar? Round your answer to two decimal places.
1.57
 ______________ is the state of matter with the least space between particles, has a definite shape and fixed volume, and keeps its shape in any container.
______________ is the state of matter with the least space between particles, has a definite shape and fixed volume, and keeps its shape in any container.
Solid
In preparation for space, flight astronaut Armstrong went over the required weight limit. What caused this?
- Static electricity
- Bag of helium gas
- Computer malfunction
- Turning on the light
Bag of Helium Gas
In her investigation, a forensic scientist measured amounts of heat, oxygen, fuel, and wood.
Which of these things is NOT matter and why?
heat -- it does not have mass / it can't be weighed
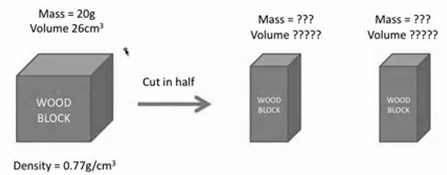 The results of cutting the block in half prove that both mass and volume are which type of physical property?
The results of cutting the block in half prove that both mass and volume are which type of physical property?
extensive
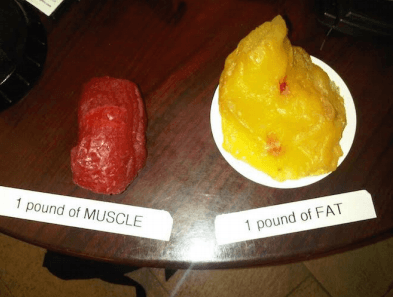
Which has more density and why?
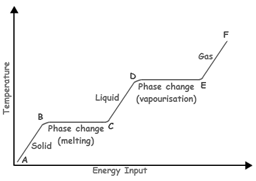
The phase change from B to C occurs due to increasing energy. This process of melting is classified as a ________.
physical change
Sugar water and kool aid are examples of a
- Element
- Compound
- Het Mixture
- Hom Mixture
Homogeneous (Hom) mixture