How many protrons does Carbon have?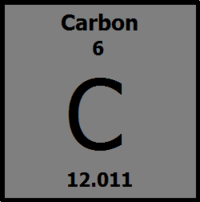
6
Elements always have the same number of...
A. Protons / B. Neutrons / C. Electrons
A. Protons
What is the name for a positively charged ion?
Cation
Isotopes share the same number of protons but have different number of...
Neutrons
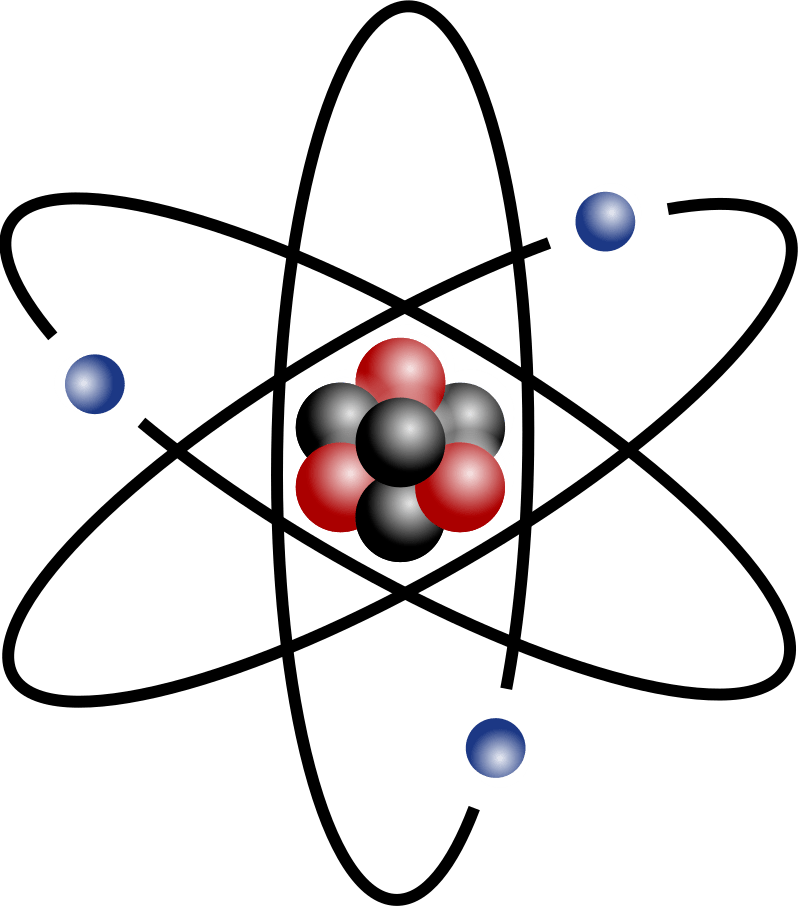 What is found in the nucleus of an atom?
What is found in the nucleus of an atom?
Protons and neutrons
Which group is the Noble Gases?
Water is made up of what 2 elements?
Hydrogen and (2) Oxygen
What is the name for a negatively charged ion?
True or false: All Carbon Isotopes will have the same number of protons?
True
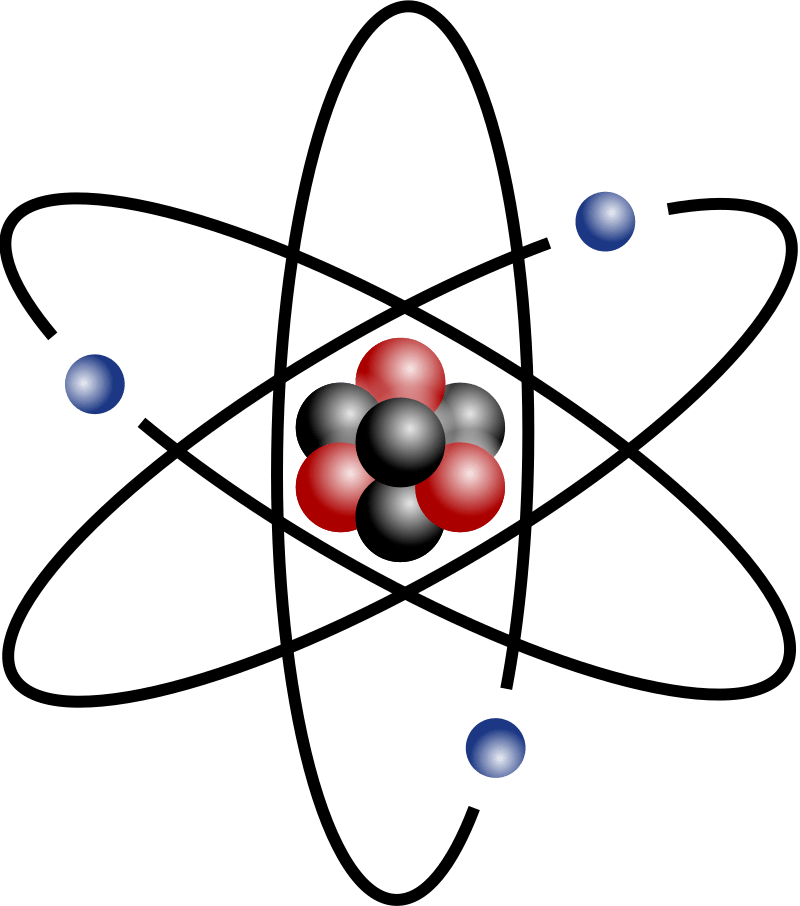 Name the 3 sub atomic particles
Name the 3 sub atomic particles
Protons, neutrons and electrons
What dies the atomic number on a periodic table tell us?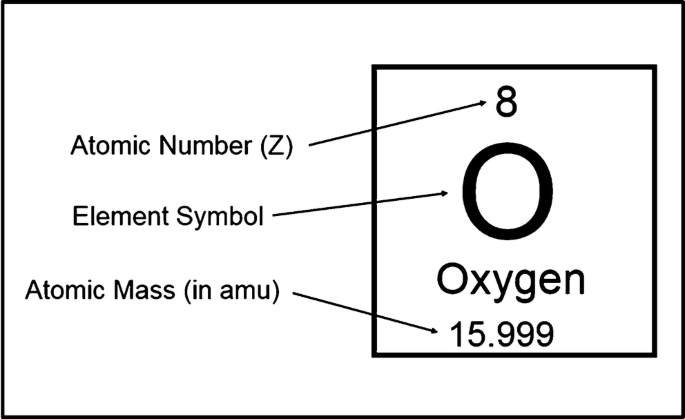
The number of protons.
What is Au the Chemical symbol for?

Gold
True or false: Ions are created when an atom gains or loses a proton.
False - it's when they lose an electron
By adding neutrons, how does this impact an atom?
It increases its atomic mass (changing it's physical properties, not its chemical ones). It is also more likely to be unstable (radioisotopes).
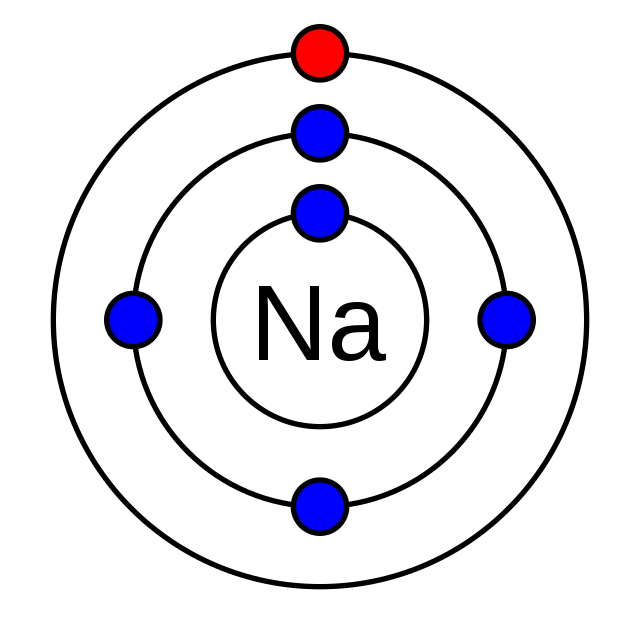 What is the scientific name for the outer shell of electrons?
What is the scientific name for the outer shell of electrons?
Valence shell
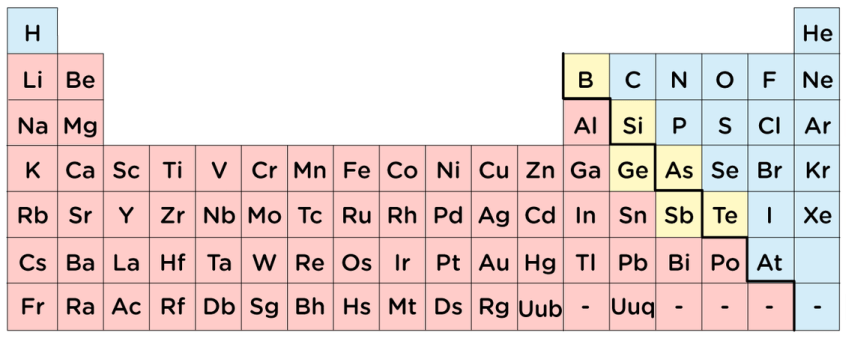 What colour are the metals on this periodic table?
What colour are the metals on this periodic table?
Red
What element has an atomic number of 1?
Hydrogen
If an uncharged atom gains an electron, it will become...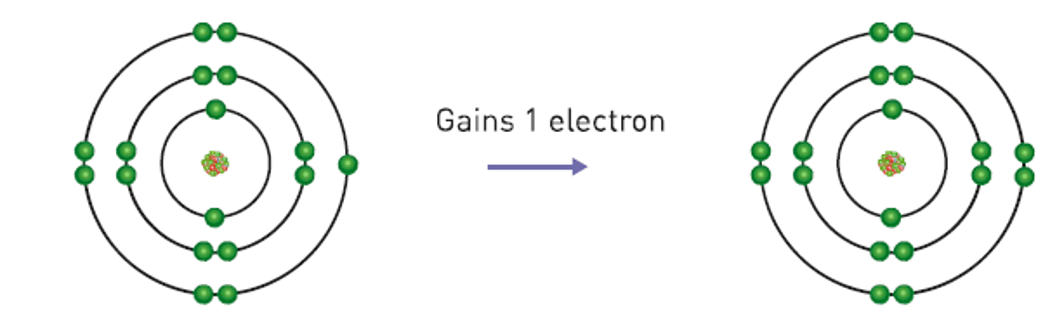
A cation (negatively charged)
Carbon has an atomic number of 6. How many protons does Carbon-13 have?
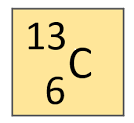
7
Explain why sodium reactive?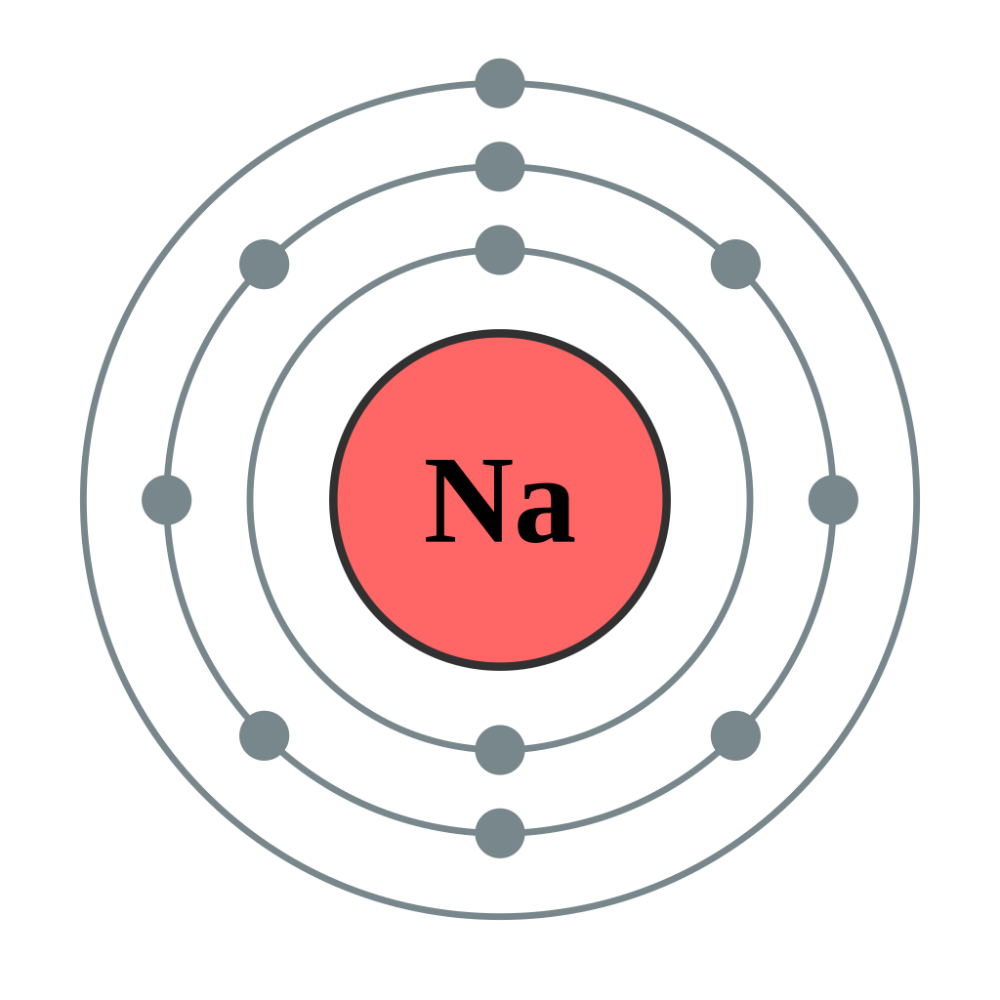
It has one electron on its outer shell.
What is the name of the rows? (shown by the different colours below).
Periods
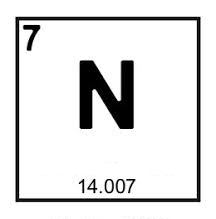 'N' is the chemical symbol for what element?
'N' is the chemical symbol for what element?
Nitrogen
Is flourine likely to gain or lose an electron? (2,7)
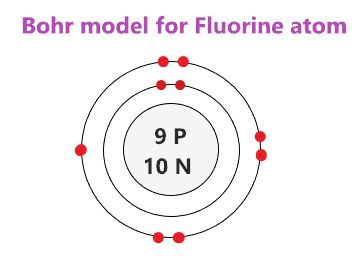
Gain an electron (to fill its outer/valence shell)
What Berylium isotope is most common?

Berylium-5.
9.0122 rounded to the nearest number is 9. 9-5=4.
What is the maximum number of electrons in the first shell?
 Why is the mass of the elements not a whole number? (e.g. - 47.867 for Ti).
Why is the mass of the elements not a whole number? (e.g. - 47.867 for Ti).
Because of the different isotopes (which have different masses).
What is the chemical symbol for Silver?

Ag
Chlorine has an electron configuration of 2,8,7. Will it become positively or negatively charged?
Negatively charged (will gain an electron)
How many neutrons does Titanium have?

26. (48-22)
How do we calculate the mass of an atom?
The number of protons and neutrons
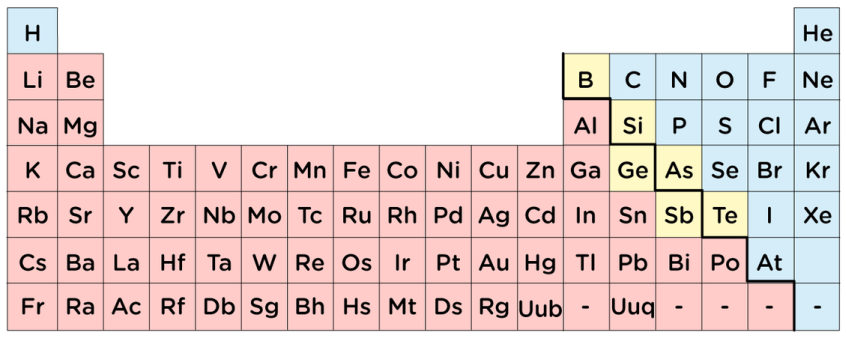 What is the name of the yellow group?
What is the name of the yellow group?
Metalloids
Which of the following is not an element?
A. Zirconium B. Einsteinium C. Pluton D. Ytterbium
C. Pluton
Calcium has an electron configuration of 2,8,8,2. Will it become positively or negatively charged?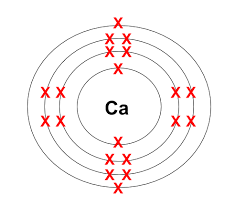
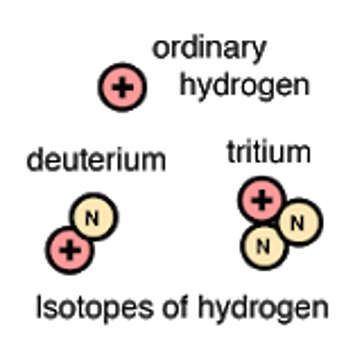
Name one of the isotopes of Hydrogen.
Deuterium (Hydrogen-2) and Tritium (Hydorgen-3)