What is an atom?
a very small piece of matter
Which of the following is a physical property.
color flammability location
What is mass?
the amount of material something is made of
What is a homogeneous mixture?
made of pure substances that are uniformly mixed. Can dissolve into each other.
 This is an example of what type of molecule.
This is an example of what type of molecule.
solid
What is an element?
Matter made of only one kind of atom.
Which of the following is a chemical property?
level smell flammability
What is matter?
Anything that has mass and takes up space
What are heterogeneous mixtures?
made of pure substances that are not uniformly mixed. Can separate and properties are different.
The difference between a liquid molecule and a gas molecule is?
A liquid molecule is more tightly packed and particles float over each other. Gas molecules are not packed and freely float.
What are the three particles in an atom?
Protons, Neutrons, and electrons
Define physical properties.
characteristics of an object that can be observed and measured
What is a property?
a characteristic that defines a substance.
Solvents and Solutes are part of what type of mixture.
homogeneous
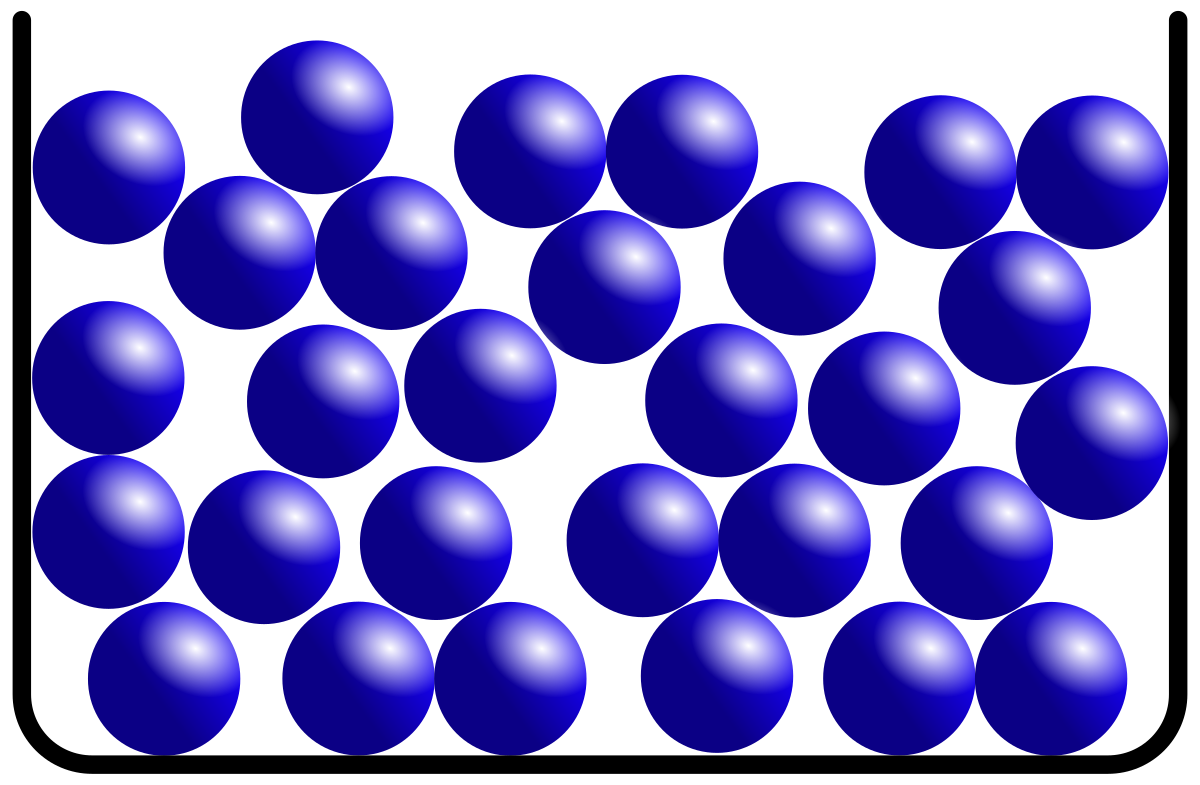 This is an example of what type of molecule?
This is an example of what type of molecule?
liquid
What are the characteristics of metals?
solid, shiny, conduct electricity, shape able, melt able,
Define Chemical property.
characteristics of that describe how one substance reacts with another
What 2 measurement devices are used to determine the mass of a solid and the volume of a liquid?
A balance measures the mass of a solid. A graduates cylinder measures the volume of a liquid
Suspensions are types of heterogeneous mixtures where the particles settle out.
true or false
true
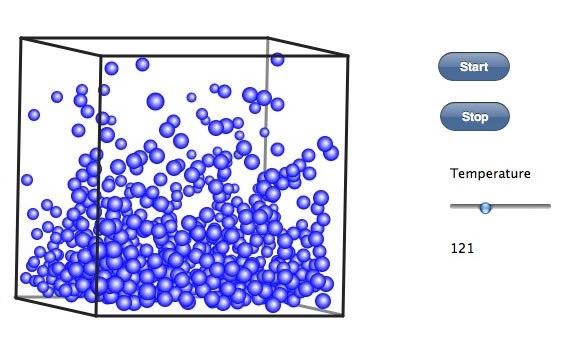 This is an example of a process where a molecule evaporates turning from ____ to _____. Fill in the blanks.
This is an example of a process where a molecule evaporates turning from ____ to _____. Fill in the blanks.
liquid to gas
What are the characteristics of non metals?
solid, liquid, or gas, dull, breakable, low electric conduction.
What are the physical and chemical properties of a piece of wood?
floats rough brown or grey flammable porous
What is volume?
the amount of space an object takes up.
What is an example of a homogeneous mixture?
miralax and water, lemonade mix and water, chocolate powder and milk.
Define Plasma
4th state of matter. caused by a reaction between heat and electricity. Found in fluorescent bulbs.