What are the three most common states of matter on earth?
Solid, liquid, gas
A column on the periodic table is called a
Group
The positively charged subatomic particle in an atom is the
proton
An element's identity or type is determined by the number of __________ it has
protons
A neutral atom has the same amount of ____ and _____
protons and electrons
Which state of matter takes the shape of its container but has a fixed volume?
Liquid
Elements in group 18 (or 8A) are called
noble gases
The negatively charged subatomic particle in an atom is the
electron
An element's atomic number is 26. What is the element
Fe or iron
Which is NOT a property of metals?
a. shiny c. brittle
b. high melting point d. conducts electricity
C--metals are malleable, not brittle
In this state of matter the atoms vibrate constantly but are held in place giving a fix shape and volume
Bat for an extra point (only if correct)
Solid
Elements in group 1 (or 1A) are called
alkali metals
The subatomic particle with no charge (neutral) in an atom is the
Shoot for 1 extra point if correct
neutron
How many PROTONS does aluminum have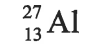
13
Carbon-12 and Carbon-14 are _______. The 12 and 14 refer to their _____________
Must answer BOTH correctly!
Carbon-12 and Carbon-14 are ISOTOPES. The 12 and 14 refer to their MASS or MASS NUMBER
The atoms in this state of matter are far apart with high kinetic energy (the atoms are moving fast). It does not have a fixed shape or volume
Gas
Elements in group 2 (or 2A) are called
alkali earth metals
Which subatomic particles are found in an atom's nucleus
Protons and neutrons
How many NEUTRONS does potassium have?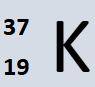
18 (37-19)
Zendaya wants her top to match her purple shoes. She's not sure what shade of purple to use so she takes 5 pieces of white cloth and dyes them various shade of purple until she finds a shade that matches her shoes. What is the independent variable?
The different shades of purple
example: amethyst, lavender, lilac, mauve, heliotrope
All matter can be divided into two categories. What are they?
1. Pure substances (elements and compounds)
2. Mixtures
The elements in group 17 (or 7A) are called
Football throw for extra point if correct
halogens
Which subatomic particle is 1000's of times smaller than the others?
electron
How many neutrons does Lead-204 (or Pb-204) have?
122 (204 - 82)
_________ must make predictions that can be tested and supported or potentially proven wrong
Wheel barrow for extra if you got it right
Hypotheses