This state of matter has a definite volume and a definite shape.
What are solids?
This picture represents what type of heat transfer?
What is radiation?
This subatomic particle is found in the nucleus and has no charge.
What are neutrons?
Name best physical property to separate a needle in a haystack.
What is magnetism?
The amount of mass in a given volume describes what physical property?
What is Density?
What is the phase changed called when a liquid turns into a solid?
What is Freezing?
What is the primary method of heat flow through a solid?
What is conduction?
In each group on the periodic table the elements do what?
What are share similar properties?
Being able to dissolve salt into water is a description of what physical property of salt?
What is solubility?
Describe the charges of each subatomic particle.
What are protons are positive,neutrons are neutral/ no charge and electrons are negative.
When a substance goes from a liquid to a gas, what must happen to the thermal energy?
What is the thermal energy must increase?
Heat flows from.
What is hot to cold?
As you go across a period what is the trend?
What is adding a proton?
The amount of matter in an object describes what physical property?
What is mass?
Find the density of a cube that has mass of 100g and a volume of 2 cm?
What is 12.5 g/cm3?
This picture represent the movements of gas particles. Describe how the particles of a solid would look and move.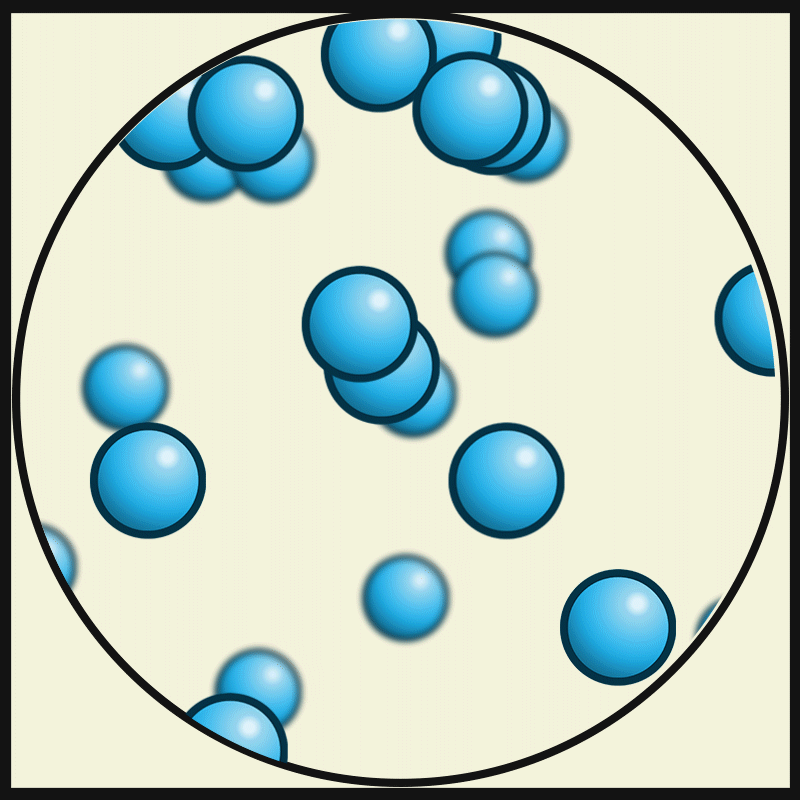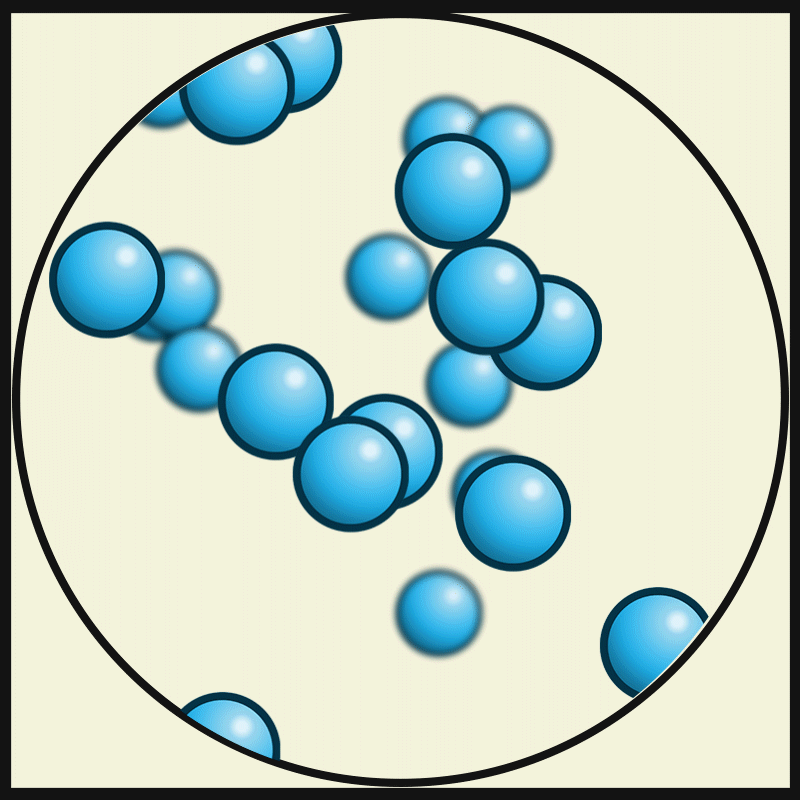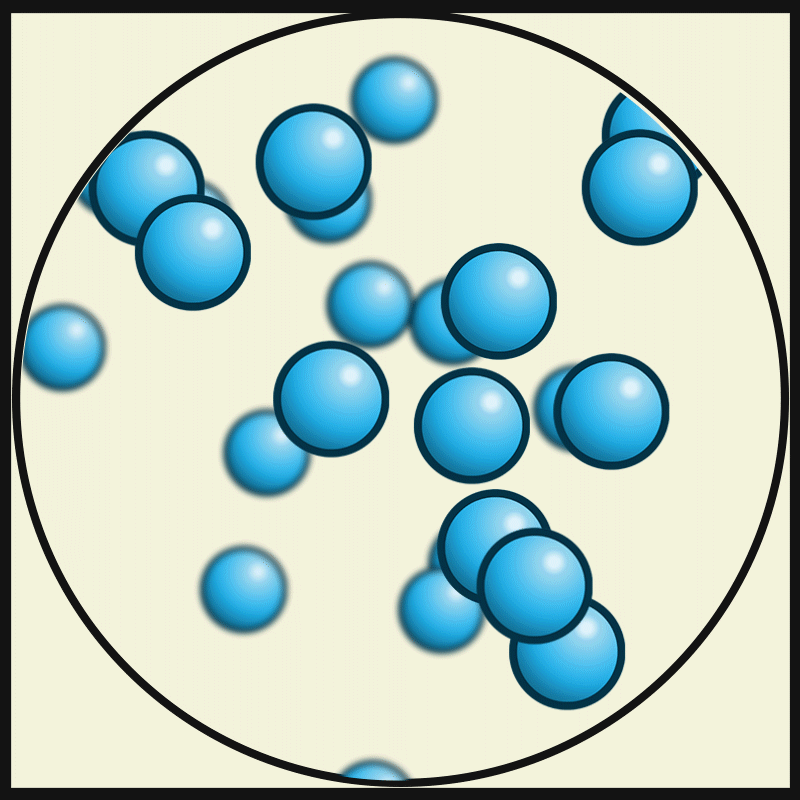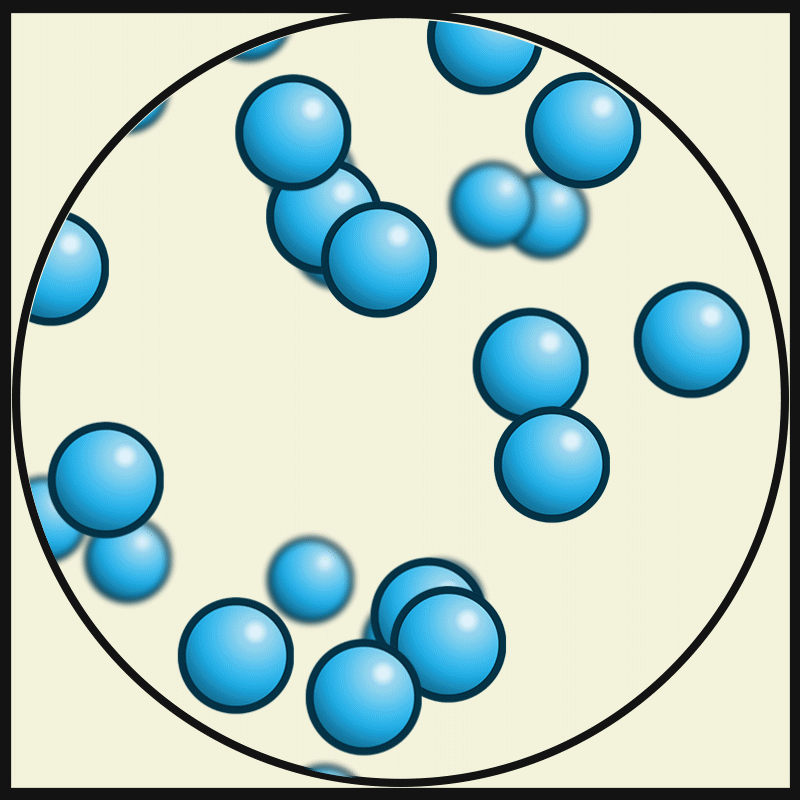
What is closely packed and vibrating?
Describe thermal equilibrium.
What is the point at which two temperatures reach and equal temperature?
Find the number of neutrons for a Silicon atom.
What is 14?
Describe the difference between physical and chemical properties.
What is, physical properties describe a characteristic of a substance without changing it into another substance and chemical properties describe a characteristic of a substance by changing into another substance.
If you were on a different planet, how would your mass and weight compare to that on Earth?
What is Mass would be the same and Weight would change?
The random erratic motion of particles in a liquid or a gas is evidence of describes what theory?
What is the Kinetic Molecular Theory?
Describe the difference between heat and temperature.
What is heat is the transfer of thermal energy and temperature is the measurement?
Looking at this model, determine the atomic number.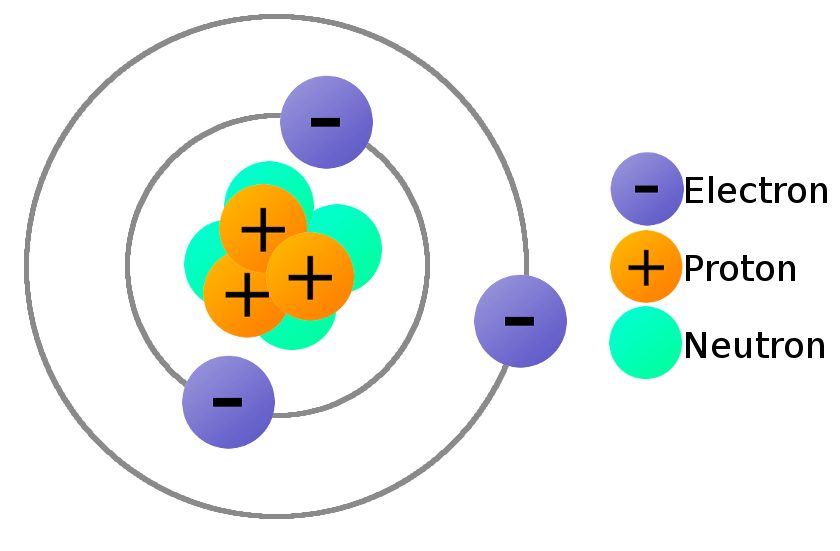
What is 3?
Find the density of an object if the mass is 25 grams and the volume is 10 cm3
What is 2.5 g/cm3 ?
Out of all of the subatomic particles _______ is the least massive.
What are electrons?