mass
This state of matter has a fixed shape and a fixed volume. Its particles are packed tightly together.
solid
A _______ is a change in matter that involves the substance going from one state (phase) to another or changing its properties (like size or shape).
physical change
The _________ is a principle that states that matter cannot be created or destroyed. It can only change forms.
conservation of matter
Name two materials that have reflectivity.
mirror, aluminum foil, surface of a lake, shiny minerals like pyrite
________ is the amount of space taken up by an object.
volume
2 liters of water are in each of the containers below. Describe the liquid's shape and volume.
Liquids have a fixed volume but not a fixed shape. Particles are free to flow around and take on the shape of their container. The volume is the same for both containers, but the shape is different.
A ______ is a change in matter that causes a new substance with new properties to be formed.
chemical change
Mark has an ice cube in a cup that weighs 5 grams total. He places the cup in the sun and the ice cube melts. He weighs the liquid water inside the cup. What will the mass be, in grams?
5 grams
Name three objects or materials that will attract a magnet.
Objects like metal pins, paperclips, nails, scissors, staples, etc.
Anything steel, brass, or iron
Which of the following items ARE matter?
pencil table electricity salt
heat baby powder water
pencil, table, salt, baby powder, water
Which state of matter can be compressed inside a closed container?
gas
Which TWO examples below are physical changes?
Sugar dissolves in water.
Wood burns in a fire.
Mold forms on a piece of old cheese.
Ice cubes melt when removed from the freezer and placed in warmer air.
Sugar dissolves in water and ice cubes melt
The mass of a jellybean and water is 225 grams. The jellybean is placed in the water and it dissolves after a few minutes. What will the mass be after dissolving?
225 grams
Heat passes easily through some objects or materials. This property is called ____________.
thermal conductivity
Which of these items are NOT matter?
rocks light steam milk
sound waves dust magnetic force
light, sound waves, magnetic force
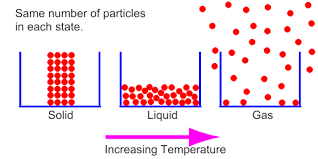
Which particles are moving the fastest? Solid, liquid, or gas?
gas
Which TWO examples below are chemical changes?
Food being digested in your stomach
A popsicle melts in the hot sun
Water boils on a hot stove
Vinegar and baking soda react to form a gas
Food being digested in your stomach and vinegar and baking soda react
 Sheila weighs her experiment materials on a scale:
Sheila weighs her experiment materials on a scale:
vinegar: 12 grams
baking soda: 5 grams
balloon: 1 gram
bottle: 15 grams
Then she makes a mixture of the vinegar and baking soda inside the bottle, and her balloon inflates with gas. What will the weight of her experiment be after this reaction is completed?
33 grams
Name two conductors of electricity and two insulators of electricity.
Effective electrical conductors:
• Many metals such as copper, silver, steel, iron, brass
Effective electrical insulators:
• rubber, glass, wood, and plastic
Which type of matter has the largest VOLUME?
a marble
a planet
a hamburger
a crayon
a mountain
a planet
Give an example of a solid, liquid, and a gas.
Answers will vary.
liquids- juice, soda, lava, honey, drinking water
solids- table, pencil, dust, ice
gas- oxygen, helium, carbon dioxide
Describe what a phase change is and how they are caused
It is a change from one state (solid, liquid, or gas) to another. Adding or removing heat energy causes phase changes.
Tom places salt in a cup of water. After 3 minutes and stirring this mixture, the salt disappears. Tom says it is gone forever! But Madeline says it dissolved and is still there. Who is correct? How could they prove it?
Madeline is correct- the salt dissolved. They could weigh the salt water before and after dissolving to see that the mass is the same. Or they could let the water evaporate and the salt would remain in the cup.
 Based on their physical properties, name one way these two items are the same and one way they are different.
Based on their physical properties, name one way these two items are the same and one way they are different.
Answers may vary.
Same shape
Different colors, textures, size, mass