What is the Mnemonic Device that we use to perform measurement conversions?
King Henry Died Unhappy Drinking Chocolate Milk (KHDUDCM)
Nonzero digits in a number are always significant. True or False?
True
What are the two units used in Chemistry to describe temperature?
Celsius (C) and Kelvin (K)
Which of the three phases of matter, does the particle diagram below represent?
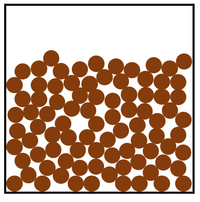
It represents a liquid.
Which type of matter is composed of two or more different elements chemically combined in a fixed ratio?
(a) a solution
(b) a compound
(c) homogeneous mixture
(d) heterogeneous mixture
(b) a compound
An object has a density of 2.33 g/cm3. This is an example of a
(a) physical property (b) chemical property
(c) physical change (d) chemical change
(a) physical property
What prefix does the "C" represent in the (KHDUDCM) Mnemonic Device?
"Centi-"
The zereos at the end of a number are significant if there is no decimal point present. True or False?
False. The zeroes at the end of a number are only significant if there is a decimal point present.
In order to solve for a mass when given a density and a volume, you have to multiply the density by the volume. True or False?
False. In order to find the mass using the density volume, you have to divide the density by the volume.
What is the name of the phase change that occurs when a gas turns into a liquid?
Condensation
Which of these types of matter would best describe air?
(a) An element
(b) A heterogeneous mixture
(c) A compound
(d) A homogeneous mixture
(d) A homogeneous mixture
Which of these statements describes a chemical property of iron (Fe).
(a) Iron is a silver, shiny metal
(b) Iron is malleable
(c) Iron has a density of 7.8 g/cm3
(d) Iron can react with oxygen to form rust
(d) Iron can react with oxygen to form rust
How many Liters of soda is 256 milliliters???
0.256 L
A student weighs a piece of filter paper on a balance and the reading is 0.0450 g. How many significant figures is in this reading?
Three Significant Figures (3)
Ethanol has a boiling point of 78.40 C. What is this temperature in Kelvin?
(a) 351.4 K (b) 194.6
(c) 273 K (d) -194.6
(a) 351.4 K
When performing heat calculations, when do you use the formula q=mHf?
You use this formula when a substance is either melting or freezing.
Which of these substances can be decomposed (broken down) further?
(a) Iron (Fe) (b) Ammonia (NH3)
(c) Chlorine gas (Cl2) (d) Oxygen gas (O2)
(b) Ammonia (NH3)
Which of these processes represents a physical change?
(a) dissolving alka seltzer in water
(b) mixing baking soda and vinegar
(c) sugar dissolving in water
(d) making pancakes on a griddle
(c) sugar dissolving in water
A student measured the weight of a beaker and the reading on the balance was 28.27 grams. The balance is able to measure to the nearest:
(a) Tens (b) Tenths
(c) Hundredths (d)Thousandths
(c) Hundredths
EXPLAIN the difference in significant figures between these two values:
1,200 and 12.00
1,200 only has two significant figures because there is no decimal point present. 1.200 has four significant figures because there is a decimal point present.
Gold has a melting point of 1,337 Kelvin. What is this temperature in Celsius?
1,0640C
The heat of vaporization of a liquid is 1344 J/g. What is the minimum number of joules of energy needed to change 40.0 g of this liquid to vapor at the boiling point?
Use q = mHv
40 x 1344 = 53, 760 Joules
What type of mixture does the particle diagram represent?
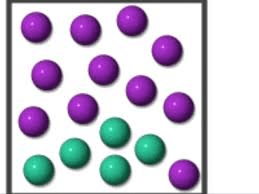
A heterogeneous mixture.
Which of these processes represents a chemical change?
(a) a candle is burning
(b) a balloon is being inflated with air
(c) a piece of copper is being pulled into wire
(d) gold is melted and then molded into a piece of jewelry
(a) a candle is burning
A drink of Arizona usually contains 15 mg of sugar. How many kilograms of sugar is this?
YOUR ANSWER MUST BE IN SCIENTIFIC NOTATION.
1.5 X 10-5 kg of sugar
Come up with a 5 digit number greater than 1 that only has three significant figures.
The first three numbers should be nonzero and then two zereos follow. There should be no decimal point present.
If a 96.5 g piece of aluminum has a density of 2.7 g/cm3 , what is the volume of the aluminum in cm3?
(a) 260.5 cm3 (b) 0.0279 cm3
(c) 35.7 cm3 (d) 3.57 cm3
(c) 35.7 cm3
If it takes 6270 Joules for a certain mass of liquid water to go from 20C to 80C. What is the mass of the liquid water rounded to nearest whole number?
(a) 60 g (b) 104.5 g (c) 15 g (d) 25 g
(d) 25 g
Which of the diagrams below are considered to be pure substances?
Diagrams A, B and C.
Oxygen is a colorless and odorless gas that makes up about one-fifth of the atmosphere of the Earth. Oxygen is usually found as a molecule made up of two atoms. Oxygen is highly reactive with metals such as sodium (Na) and potassium (K).
IDENTIFY ONE PHYSICAL PROPERTY AND ONE CHEMICAL PROPERTY FROM THE INFO GIVEN ABOVE.
PHYSICAL PROPERTIES
-Colorless and Odorless
CHEMICAL PROPERTIES
-Oxygen is highly reactive with metals