How is density different from Metals to Nonmetals?
metals have high density
Nonmetals have low density
What type of change does combustion indicate
chemical
dew drops forming on a leaf is an example of
cohesion
What pH numbers indicate a base
8-14
What is the function of your integumentary system?
act as a barrier, protect
The word malleable describes what classification on the periodic table
metals - cam be hammered or rolled into shape
Evaporation describes what type of change
physical
A drop of water falling down a window
Adhesion
What are at least 2 key properties of acids
0-6 pH, watery, sticky, reacts with metals, turns blue litmus paper red, taste sour, releases H ions
What is the function of a ribosome
make protiens
Semi conductor describes what classification on the periodic table?
metalloid - can conduct electricity under certain situations
Define precipitate.
2 liquids mixed that form a solid
the property that would keep a paperclip floating on top of a beaker of water
Surface tension
What pH numbers indicate a acid
0-6
What are the features seen at a convergent boundary, must have all
volcano, trench, mountain
What does brittle mean and what does it classify on the periodic table
breaks easily - nonmetals
can you reverse a physical change
yes physical changes are reversible

This walking water experiment demonstrates what property
capillary action
What are 2 properties of bases?
slippery, used in cleaners, turns red litmus paper blue
Define autotroph
an organism that makes its own food using PHOTOSYNTHESIS
What is the unique property about metalloids
They share properties of both metals and nonmetals
Name all 7 indicators of a chemical reaction
1. Color change 2. Temperature change 3. Bubbles / gases produced 4. Precipitate forms (new substance) 5. Light produced 6. Sound produced 7. Smell/odor
Water is a universal _______
solvent
What color does the color orange represent on the pH scale?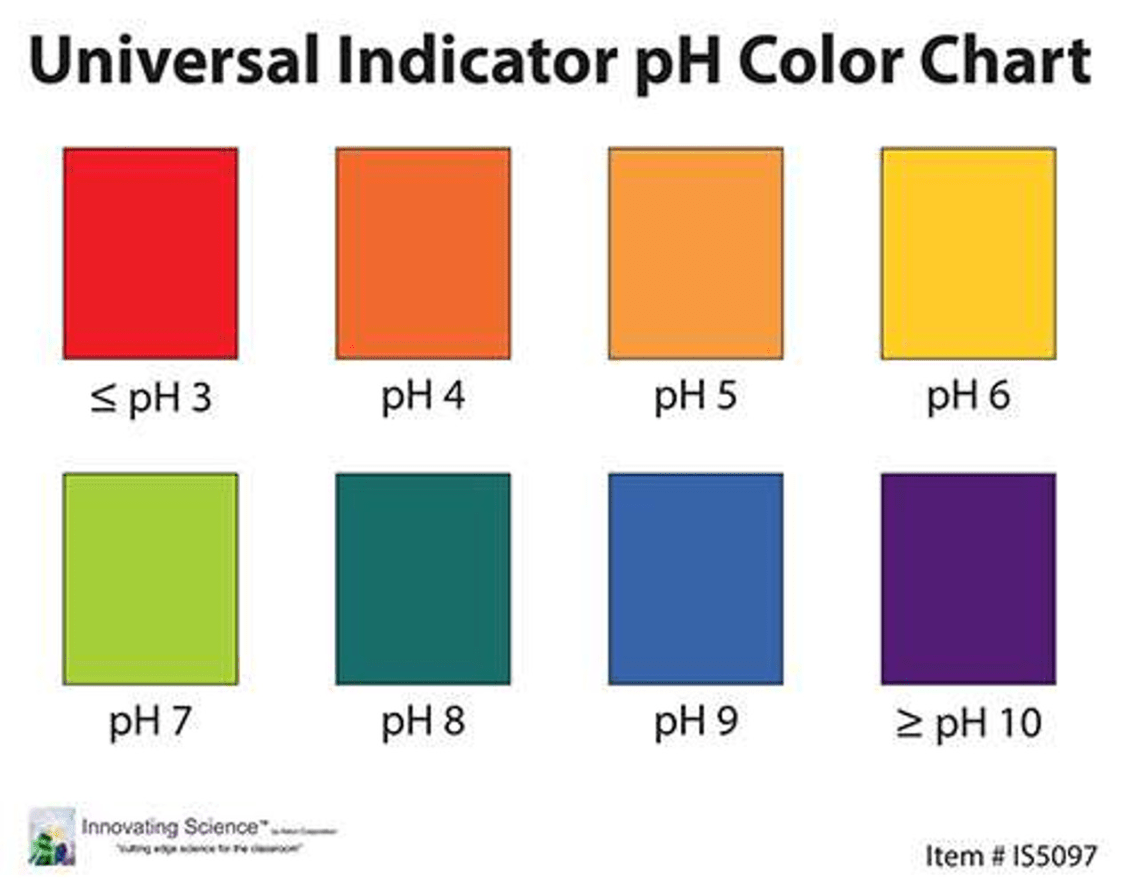
orange is an acid
name all 6 taxonomic kingdoms
Archaea, Bacteria, protista, fungi, plantae, animalia