Which element is in Period 4 and Group 2?
Calcium
Name all the subatomic particles in an atom, their charges, and where to find them in an atom.
Proton, +, Nucleus
Electron, -, Electron Cloud or Orbitals
Neutrons, no charge (o), Nucleus
Zirconium - Zr
Element
The process by which heat is transferred through the movement of a fluid (liquid or gas) due to density differences is called.
Convection
Steel Wool Rusting after being placed in Vinegar
Chemical Reaction
What is the Law of Conservation of Energy?
Energy cannot be created or destroyed, it can only be transformed between different types.
You use water to wash dirt, salt, and sugar out of a glass container, which are all easily dissolved by the water.
Water is an Excellent Solvent (Universal Solvent)
Will the following items float or sink based on their densities in Fresh Water at 4*C
Cork - 0.24 g/cm3
Concrete - 2.0 g/cm3
Lead - 11.3 g/cm3
Oak - 0.60 g/cm3
Ethyl Alcohol - 0.79 g/cm3
Aluminum - 2.70 g/cm3
Cork - Float
Concrete - Sink
Lead - Sink
Oak - Float
Ethyl Alcohol - Float
Aluminum - Sink
A water strider insect seems to be walking or gliding effortlessly across the surface of a still pond.
Surface Tension
How many protons, electrons, and neutrons are in a neutral atom of Potassium (K)?
Protons - 19
Neutrons - 20
Electrons - 19
Define a compound
A combination of two or more elements that are chemically bonded together.
Aluminum - Al
Element - Pure Substance
Putting a cold spoon into hot soup would transfer energy from the ____________________ to the __________________________.
Soup to the Spoon. Thermal energy always moves from Hot to Cold.
Invisible Ink - Lemon Juice Ink changes color and becomes visible when placed under a heat lamp
Chemical Reaction
A fully charged battery sitting on a shelf represents stored energy. What specific type of potential energy is stored within the battery?
Chemical Potential Energy
You fill a glass of water completely to the rim, but you are able to add a few more drops before the water spills over, creating a dome shape.
Surface Tension
A piece of metal has a mass of 135 g. When placed in a graduated cylinder containing water, the water level rises from an initial volume of 40.0 mL to a final volume of 55.0 mL. What is the density of the metal? **Solve for the Volume first**
D = M/V
Solve for Volume - 55 mL - 40 mL = 15 mL (1 mL = 1 cm3)
135g / 15cm3 = 9 g/cm3
What is the Law of Conservation of Matter?
Matter cannot be created or destroyed, it is only changed and rearranged.
If an element is shiny, conducts electricity and heat well, and is malleable, it probably belongs to which periodic family? (Cu, Ti, and Fe are members of this family)
Metals
Explain the particle motion of atoms all 3 phases and describe their shape and volume.
Solid - particles vibrate in place - definite shape and definite volume
Liquid - particles flow over one another - definite volume and NO definite shape
Gas - particles expand to fill any given space, moving quickly - NO Definite Shape or Volume
Mixture - Nitrogen (N2), Oxygen (O2), Argon (Ar), Carbon Dioxide (CO2), etc.
Energy transferred through direct contact between two objects or substances is known as:
Conduction
Ice melting into water and being placed back into the freezer and refreezing.
Physical Changes
A hydroelectric dam converts the energy of falling water into electrical energy. What is the fundamental energy transformation that takes place at the turbine?
Gravitational Potential Energy -> Kinetic Energy -> Electrical Energy
Water is drawn from the roots of a tall tree all the way up to its highest leaves against the force of gravity.
Capillary Action
Solve for the Sphere's mass: D = M/V
Sphere's Density (D) =2.5 g/cm3
Sphere's Volume (V) =48 cm3
2.5 g/cm3 = m/48 cm3 -> 2.5 g/cm3 x 48 cm3 = Mass
Mass = 120 grams
The upstairs in a house is usually warmer than downstairs. This is due to this type of heat transfer _________________________________.
Convection
An element has 38 protons and 38 electrons. What is the element's name and chemical symbol, and what is the mass of this element?
Strontium - 87.62 AMUs
Explain why not all molecules are compounds.
Compounds must contain combinations of 2 or more different elements. - Carbon Dioxide - CO2
Molecules can be combinations of the same element - Breathable Oxygen O2
Baking Soda - NaHCO3
Compound
Mittens or gloves keep you hands warm because they are good ________________________.
Thermal Insulators
Crumpling a Sheet of Paper
Physical Reaction
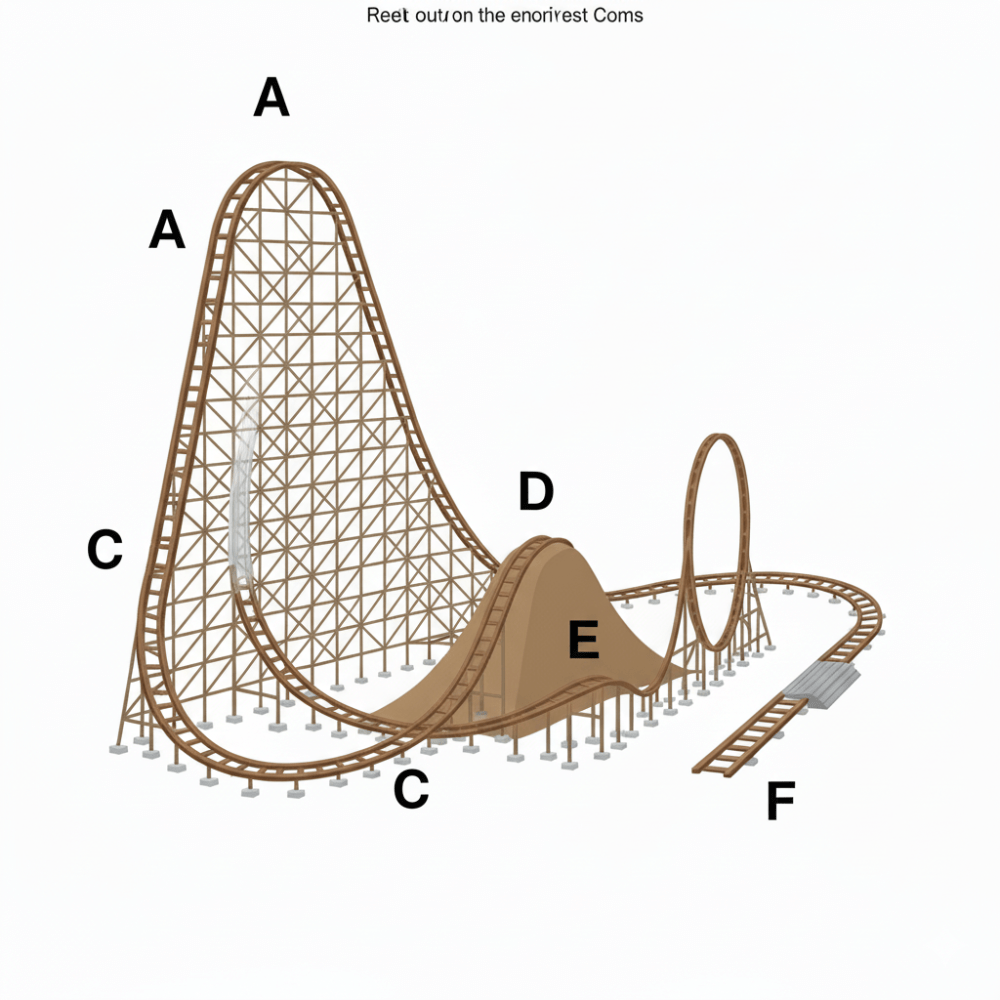
Identify what is happening at each of the specified areas along the track - Between the 2 A's, Between C's, at D, and F.
Between C's Kinetic Energy is at it's Maximum
At D - Less Potential Energy but, less than the first hill
F - Used all of it's KE and PE - Stopped
After a rain shower, small droplets of water cling to the side of your car window or a spiderweb, sticking to the surface rather than falling straight down.
Adhesion
A solution has a mass of 85 g and a total volume of 65 mL. What is the density of the solution in g/mL?
D = Mass/Volume
D = 85 g / 65 mL
Density = 1.3 g/mL
What type of energy is transferred through electromagnetic waves (i.e. gamma rays, x-rays, radio waves, etc)?
Radiant (Light) Energy
Name the Elements and how many atoms of each are present in the following chemical formula (pay close attention to the Coefficients and Subscripts and do not duplicate like elements - count them all together)
3CH3COOH
Carbon - 6
Hydrogen - 12
Oxygen - 6
Why is a Lava Lamp a good example of a Heterogeneous Mixture
The 2 materials in the lava lamp will settle out when it's no longer heated and are not uniformily mixed.
Brass - an Alloy of Zinc and Copper
Mixture
The heat you feel from the sun or a campfire is primarily transferred to you through:
Radiation because no physical medium is required to transmit the energy.
Water and Oil Separating after being shaken inside the same container for 2 minutes
Physical Change
Define Energy
The ability to do work or cause change
When driving in the winter, the temperature gauge in your car stays regulated, and you must add a special fluid to the radiator.
High Specific Heat
How much matter is in the volume of a given space. - Mass per unit of volume.
________________________ is the Energy of Motion
Kinetic Energy