Juice you buy from the grocery store...Which specific type of mixture
What is a homogeneous mixture?
The smallest unit of matter
ATOMS
What is a chemical change?
Calculation for density
What is the mass divided by the volume?
A substance containing 2 or more different atoms, bonded together is a
What is a compound?
Rusting is an example of what
Chemical Change
SUGAR OR SALT are pure substances or mixtures?
PURE SUBSTANCES
The two things that make up matter
What is MASS and VOLUME
Boiling water is an example of what type of change

Physical change (A change in state)
A liquid with the least density such as rubbing alcohol will do what if poured into a glass that has liquids with different densities?
What is float to the top?
Atoms of only one type are called ______________
What is elements
This measurement changes if you are on different planets
What is Weight?
Sugar Water solution Pure substance or Mixture
Mixture
This is why we use Mass instead of Matter
What is Gravity?
Sugar or Salt dissolved in water
Physical change
If a basketball has a volume of 140 cubic cm and a mass of 560 grams, what is its density?
4 grams/cm3
N2 and H2 are examples
Molecules
Mass, Matter and Density all share this
What is all 3 are NOT affected by gravity
Any element in the periodic table including Hydrogen, Oxygen, Carbon, Gold that cannot be broken down any further
What is a pure substance
A crushed can is an example of what subset of which type of property
subet: change in form or appearance
Type: Physical change
Flammability, Radioactivity, Reactions
What are chemical properties?
Knowing the density of an unknown substance will allow you to discover...
What it's identify is?
H2O or Water
What is a compound and a molecule?
During a fire what happens to all the mass of the fire and the objects burned
The mass changes form but the amount of mass after the reaction is the same amount of mass as before
Smog a fog or haze with smoke and other atmospheric pollutants. Pure substance or Mixture.
What is a Mixture?
White is Oxygen and Red is Carbon. Types/Amount of Atoms and what compound/molecule is this
What is 2 Oxygen atoms + 1 Carbon Atom, what is CO2 or Carbon Dioxide?
How is a chemical change different from a physical change?
Chemical change results in new substance with different chemical properties.
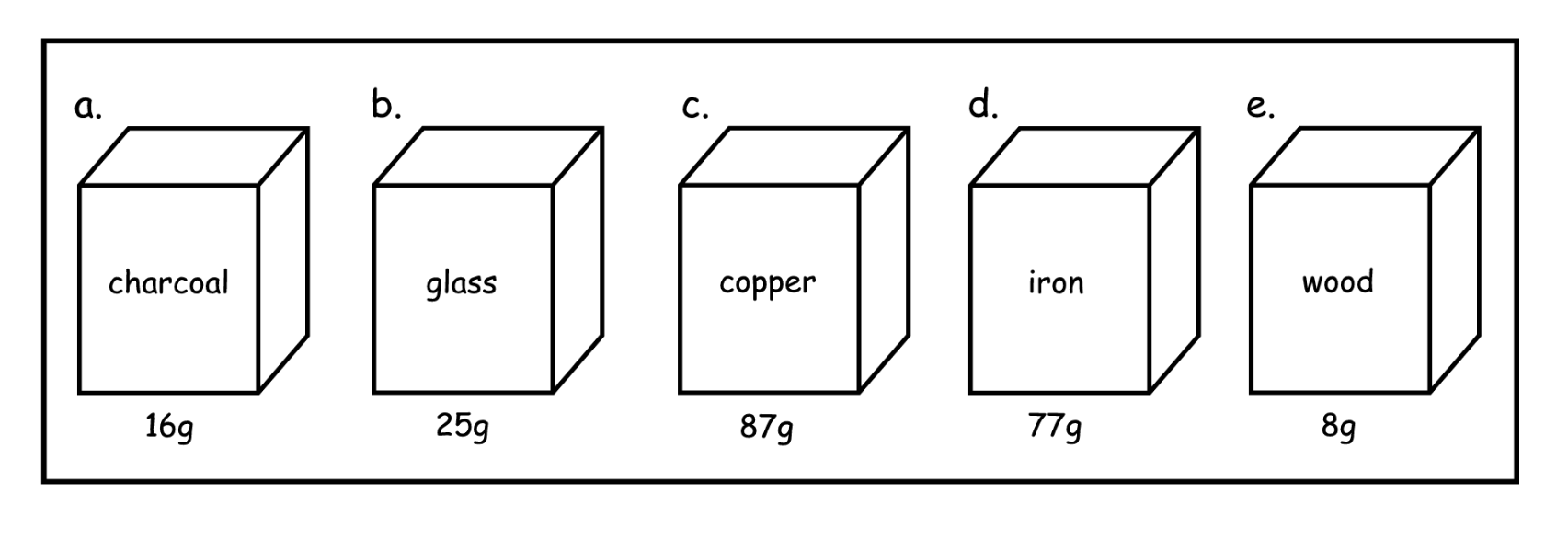
All these blocks have the same what? but different what? Which of these will float?
They all have the same volume.
All have different masses
The wood block is most likely to float to the top because it is the least dense
Diamond and Graphite
What are 2 forms of the element Carbon
Calculate density for 2 liquids. Which will sink, which will float?
Liquid 1 is 137 grams and 100 mL
Liquid 2 is 92 grams and 100 mL
Liquid 1 density is 1.37 g/mL. more dense/sink
Liquid 2 density is 0.92 g/mL, less dense/float