What is the formula for density?
Density = Mass/Volume
What is the atomic theory?
The scientific theory that matter is composed of particles called atoms
What phase change occurs when a substance cools down to its melting point?
Solid to liquid
Does mass change in cellular respiration?
No, due to the law of the conservation of mass
Is salad a type of homogenous or heterogenous mixture? Why?
Heterogenous because it is not consistent through the mixture. It has different parts that are easily seen.
Which liquid is the least dense? Which one is the most dense? Why?
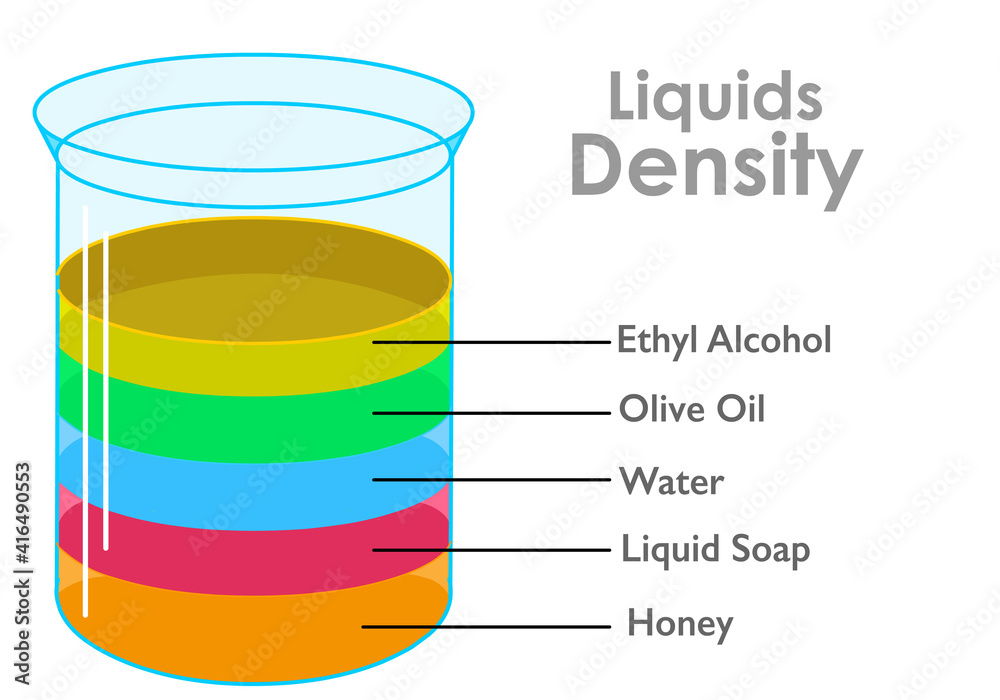
Ethyl alcohol is the least dense, because it stays at the top. Honey is the most dense, because it sinks to the bottom.
What are the two parts of the structure of an atom, and in which is most of the mass located in an atom? Why?
The nucleus and cloud of electrons. The nucleus is where most of the mass is located, because the neutrons and protons are much heavier than electrons.
What phase change occurs when a substance cools down to its boiling point?
Liquid to gas
Is photosynthesis a chemical change or a physical change? Why?
The chemical reaction that takes place in photosynthesis process involves a reaction between carbon dioxide, water, and light to form glucose and oxygen. Because a new product is formed in this process, photosynthesis is a chemical change.
Define acid, base, and neutral
Acid: any substance with a pH < 7
Base: any substance with a pH > 7
Neutral: any substance with a pH = 7
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 mL. The mercury used to fill the cylinder weighs 306.0 g. From this information, calculate the density of mercury
13.6 g/mL
Protons are positive, neutrons are neutral, and electrons are negative
Does the amount of mass change in a chemical change? In a physical change?
No, the law of conservation of mass states that mass cannot be created nor destroyed
What is the equation of photosynthesis?
6CO2 + 6H2O + energy --> C6H12O6 + 6O2
carbon dioxide + water + energy --> glucose + oxygen
What are two elements that would behave similarly to chlorine?
fluorine, bromine, iodine, astatine, tennessine
An apple weighs 100g and has a volume of 80cm^3. What is its density? Does it float in water?
0.8g/cm^3
Yes, it floats in water because it has a lower density than water, which equals 1g/cm^3
How are elements with similar properties grouped in the periodic table?
Columns/groups. They are also called families because of their similar chemical behavior. All the members of a family of elements have the same number of valence electrons and similar chemical properties.


What is the equation of respiration?
C6H12O6 + 6O2 --> 6CO2 + 6H2O + energy
glucose + oxygen --> carbon dioxide + water + energy
Why does a wire have copper on the inside and rubber on the outside?
Copper is an excellent conductor of electricity, while rubber is an insulator that does not allow the flow of electricity. This protects the circuit and allows people to safely handle wires.
What is the weight of the ethyl alcohol that exactly fills a 200.0 mL container? The density of ethyl alcohol is 0.789 g/mL.
157.8g
Explain the difference between elements vs. compounds vs. mixtures
An element contains just one type of atom. A compound contains two or more different atoms joined together. A mixture contains two or more different substances that are only physically joined together, not chemically. A mixture can contain both elements and compounds.
What 3 factors can help increase the rate of a reaction?
Increasing temperature, concentration, pressure
What is the relationship between photosynthesis and respiration?
The glucose and oxygen made by photosynthesis is used in cellular respiration, and the carbon dioxide made in cellular respiration is used in photosynthesis.
28.5 g of iron is added to a graduated cylinder containing 45.50 mL of water. The water level rises to the 49.10 mL mark, from this information, calculate the density of iron.
Hint: You can find the volume of an irregular solid using water displacement
Volume of iron: 49.10 mL−45.50 mL= 3.60 mL
Density of iron: 28.5 g / 3.60 mL= 7.92 g/mL
The density of iron is 7.92 g/mL