How much space matter takes up
Volume
The primary metals that are attracted to a magnet
Steel and Iron
Fastest way to separate steel and aluminum cans/
A magnet
What is a mixture?
A combination of 2 or more ingredients.
What property of salt remains unchanged when it dissolves in water?
Taste
Mass
Physical Property
Are there any physical changes when you mix salt and water together?
If yes, what change or changes?
Yes
Texture and shape
Rigid in shape
Solid
If you add 10 mg of salt into a container with 200 mL of water then evaporate the water. How much salt will you get back?
A. 5 mg
B. 10mg
C. 200 mg
D. 0 mg
10 mg
The force exerted by magnets when they attract or repel each other.
Magnetism
The ability to send heat or electricity through matter.
Conductor
What is the best way to classify something made of metal?
Magnetic vs not magnetic
True or false: A solution is a mixture
How can you separate a solution of sugar and water?
Evaporation
Are there any physical changes when you mix plastic and steel paperclips together?
If yes, what change?
No
Takes the shape of the container, no fixed volume.
Gas
Adding heat to change a solid to a liquid.
Melting
How much matter is inside an object
Mass
Solid, Liquid, Gas
Physical State or State of Matter
How do you classify this object?

Less dense than water.
Which of the containers can use a strainer to separate?
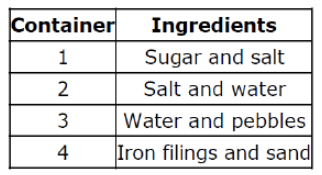
Container 3
Pebbles are like gravel and too big for the openings.
What 2 properties of salt that change when dissolved in water?
The texture and the size.
What is the definition of a physical change?
When some property of the matter changes but some properties remain.
Takes the shape of the container, fixed volume.
Liquid
Removing heat to change a liquid to a solid.
Freezing
True or False: All metals are magnetic
False
The ability to keep heat or electricity from passing through matter.
Insulator
Which material is the best conductor of heat?
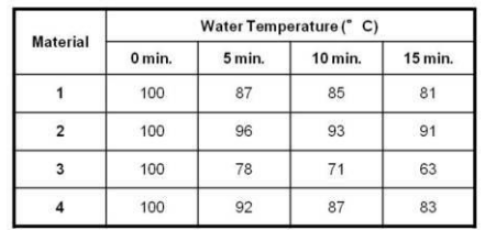
Material 3
The best conductor sent energy through it and into the air. It has the lowest temperature after 15 minutes.
What are the 4 steps to separate a container of glass beads, plastic beads, and steel beads into 3 piles?
(plastic beads are less dense than water)
1) Magnet to separate the steel beads
2) Add water to the container to make the plastic beads float
3) Skim/Scoop the plastic beads from the water.
4) Tea strainer to separate the water from the glass beads
What is the best way to describe what happens to matter when it dissolves in water?
The matter breaks into smaller pieces until it is too small to see.
Are there any physical changes when you make lemonade?
If yes, what change?
The sugar dissolves.
Adding heat to change a liquid to a gas
Evaporation
Why does a soda can that was taken out of a refrigerator develop condensation on the outside of the can?
Because the temperature of the air is warmer than the temperature inside the can.
To break down into small pieces too small to be seen.
Dissolve
Whether something sinks or floats in water
Relative Density
How do you classify a 2 metal bolts with 2 properties of matter: 1 is copper and 1 is iron.
Copper: Conductor not magnetic
Iron: Conductor is magnetic
What are the 4 steps to separate a mixture of salt, iron filings, and sand into 3 separate piles?
1) Use a magnet to separate the iron from the salt and sand
2) Pour the salt and sand into water
3) Pour the water and sand through a coffee filter and funnel
4) Evaporate the water
What is the biggest and most important difference between a mixture and a solution.
One ingredient goes through a physical change to make a solution.
Which container had at least 1 ingredient have a physical change when mixed?
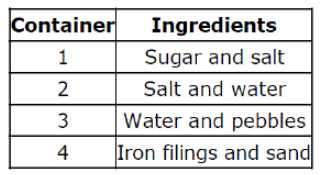
Container 2
Salt and changed size and texture.
Removing heat to change a gas to a liquid.
Condensation
When you make a solution of salt and water, is the mass of the salt conserved within the solution?
Yes. If you add a specific amount of solute (salt) the solution conserves (keeps) the mass.
example: 100 g of water + 10 g salt - 110 g solution