What does the atomic number of an element represent?
number of protons found in that atom
On a periodic table, what are rows called?
periods
An atom’s mass number is 210 and its atomic number is 85. How many neutrons does the atom have?
What element is it?
What element is in the 4th period and 12th group?
zinc
What main group of elements have solids, liquids and gases at room temperature?
non-metals
What subatomic particles make up the mass of an atom?
protons and neutrons
What do all elements in the same column have in common?
React the same way
Would metals, nonmetals, or metalloids be described as having the following properties?
luster, malleable, ductile, good conductors, donate electrons, high density, high melting point
metals
What element was named for the creator of the periodic table?
101 - Mendelevium
Which noble gas is the smallest?
Helium
The staircase follows what group of elements down the periodic table?
metalloids/semimetals
What is the name of the vertical organization of the elements on the periodic table?
group or family
What are three physical properties of non-metals?
not shiny, not malleable (brittle), not ductile, poor conductors (good insulators), gain electrons, low density, low melting point
The element is located at the 6th period and 2nd group?
What is Barium?
The ability to be made into a wire:
ductility
Which groups house the transition metals?
Groups 3-13
What is the atomic number of Calcium
20
What element is grouped with alkali metals (Group 1) even though it is not an alkali metal.
Hydrogen
What is the first element in period 3?
Sodium
What is the name of the group 2 family?
alkaline earth metals
Argon belongs to what specially named group?
noble gases
Which family (name, not number) is the most reactive metals family with one valence electron?
Alkali Metals
What is the name of group 17?
Halogens
How many electrons does a neutral atom of iodine have?
53
What does a solid do when it turns into a gas?
Evaporates
What element has the symbol Au
Gold
Who is the creator of the Modern Periodic Table?
1/2 points - which country was he from?
Who is Dmitri Mendeleev
Russia
An atom contains 14 protons, 14 neutrons, and 14 electrons. What atom is it?
Silicon
A Carbon atom has 6 Protons. What group is it in?
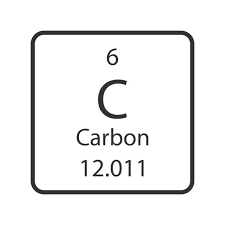
Group 14
What two elements make up table salt?
Sodium and Chlorine