of Matter
of Matter
of Matter
Mixtures
in Water
Chemical
Changes
Renee was comparing the physical properties of two objects. When she placed the objects on a pan balance with one object on each side, the side of the pan balance with Object A was lower than the side with Object B. What is most likely true about the two objects?
A. The masses of both objects are equal.
B. The mass of Object A is less than Object B.
C. The mass of Object A is greater than Object B.
D. The mass of Object B is greater than Object A.
C. The mass of Object A is greater than Object B.
Keith and Jenna are comparing two samples of matter. They make a table of the properties of each sample.
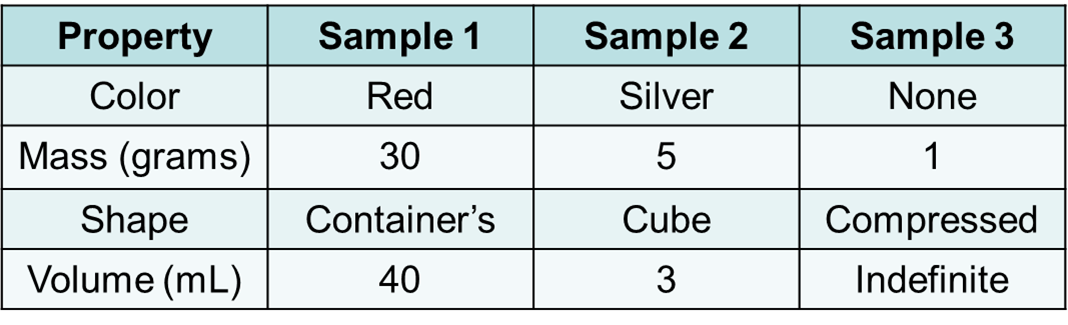
Which sample is most likely a solid?
A. Sample 1
B. Sample 2
C. Sample 3
D. None of the samples are a solid.
B. Sample 2
Jamal’s teacher gave each group a bucket full of rocks and sand. She challenged the groups to find a way to completely separate the rocks from the sand. What could Jamal’s group do to completely separate the rocks from the sand and why?
A.add water to the mixture because all rocks float
B.use a magnet to remove the rocks from the sand because all rocks are magnetic
C.run the mixture through a sieve because the smaller particles of sand will pass through the holes
D.separate the pieces according to their color because sand and rocks are very different color
C. run the mixture through a sieve because the smaller particles of sand will pass through the holes
Lucas was making dessert for his family. He added sugar to his mixture, but a lot of the sugar did not dissolve right away. What could Lucas do to make the sugar dissolve faster?
A.add more sugar
B.reduce the amount of water
C.reduce the temperature of the water
D.stir the mixture thoroughly
D. stir the mixture thoroughly
Jacob conducted an experiment to see if milk needed to be refrigerated. He placed three cups of milk in the refrigerator and three cups of milk on the kitchen counter. He concluded that milk needs to be refrigerated. Why does milk have to be kept in a refrigerator?
A.Milk tastes better when it is cold.
B.Physical changes that cause milk to spoil are slower at lower temperatures.
C.Chemical reactions that keep milk from spoiling are faster at lower temperatures.
D.Chemical reactions that cause milk to spoil are slower at lower temperatures.
D. Chemical reactions that cause milk to spoil are slower at lower temperatures.
Marcus is comparing the physical properties of a marble and a rock. He determines that the marble is smooth, the rock is rough, and they have equal masses. What would be the best way for Marcus to now find the volume of the irregular shaped objects?
A. use a pan balance and calculate the volume with gram masses
B. use a graduated cylinder of water and calculate the volume through displacement
C. use a ruler and calculate the volume by measuring each object’s sides
D. liquefy the objects and calculate the volume using a graduated cylinder
B. use a graduated cylinder of water and calculate the volume through displacement.
Desiree and Vince are comparing two samples of matter. The table below shows the properties of each sample.
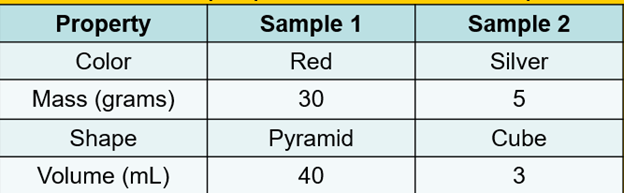
Which property provides the best evidence that both samples are solids rather than liquids?
A.Color
B.Mass
C.Shape
Volume
C. Shape
Two substances make up a mixture. The chart below shows properties of the two substances.
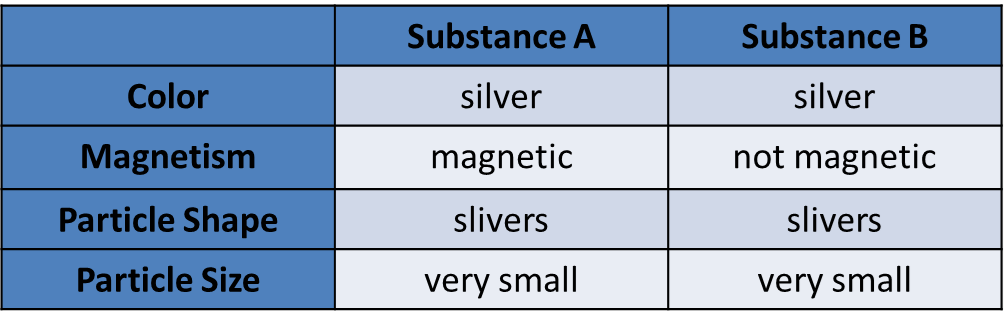
What is the fastest way to separate Substance A from Substance B?
A.Use a magnet to attract one of the substances and leave the other one.
B.Put the particles in a container of water so that one will float and one will sink.
C.Dissolve the substances in water and then evaporate the water to separate them.
D.Sift the smaller particles through a sieve to separate the bigger and smaller ones.
A. Use a magnet to attract one of the substances and leave the other one.
Four classmates were experimenting with dissolving rates of sugar. Sam placed a one gram sugar cube in a cup of room temperature water and let it sit until the sugar cube dissolved. Gary placed a one gram sugar cube in a cup of hot water and let it sit until it dissolved. Andy placed a one gram sugar cube in room temperature water and stirred until it dissolved. Richard crushed a sugar cube into pieces, placed the pieces in room temperature water, and let them sit until they dissolved. Whose sugar most likely dissolved the slowest?
A.Richard’s dissolved the slowest.
B.Andy’s dissolved the slowest.
C.Gary’s dissolved the slowest.
D.Sam’s dissolved the slowest.
D. Sam’s dissolved the slowest.
Iron becomes rust when it reacts with oxygen. Which of the following is another example of a material undergoing a chemical change to become another material with different characteristics?
A. melting ice
B. burning wood
C. freezing water
D. shattering glass
B. Burning wood
Eddie experimented to find out which freezes at a lower temperature, distilled water or salt water. The steps he used in his experiment are shown below.
Procedure:1. Put room-temp. distilled water in one beaker.
2. Put room-temp. saltwater in another beaker.
3. Place both beakers in a freezer.
4. Observe and measure the temperature at which the liquids
freeze.
What important piece of information did Eddie forget to include in his procedure?
A. the type of water being used
B. the temperature of the water being used
C. the amount of water being used
D. the brand of freezer being used
C. the amount of water being used
Each of the states of matter has specific characteristics. Which statement correctly describes one of these characteristics?
A.Solids flow from one place to another.
B.Gases have a definite volume and shape.
C.Liquids can expand and be compressed.
D.Liquids take the shape of their containers.
D. Liquids take the shape of their containers.
Mrs. Laster’s class is sorting mixtures based on their observable properties. The students were given a bag of objects and pictures and asked to sort them into groups with one similar property for each group. The table below shows how some of the students sorted their objects.
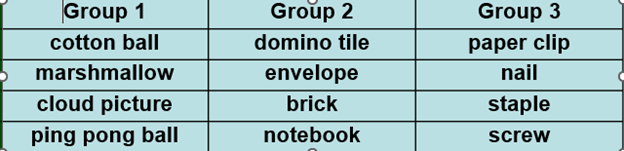
Based on the table, which of the following is most likely how Ms. Laster’s students sorted their objects?
A. Group 1 - shape
B. Group 2 - size
C. Group 3 - shape
D. Group 1 - color
D. Group 1 – color
Water is called the universal solvent because it is capable of dissolving more substances than any other liquid. Which of the following statements about water’s dissolving capabilities is true?
A.Sand settles to the bottom of a container of water and does NOT dissolve.
B.Oil settles to the bottom of a container of water and does NOT dissolve.
C.Sand floats on top of water and does NOT dissolve.
D.Salt settles to the bottom of a container of water and does NOT dissolve.
A. Sand settles to the bottom of a container of water and does NOT dissolve.
Physical changes are changes that can only change an object’s size, shape, or state of matter. Physical changes do not make something new and completely different, and can often be easily reversed. Barnie says that freezing water to make ice is a physical change. Which of the following actions best proves that freezing water to make ice is a physical change?
A.Place water in a pot, heat it, and observe it boil away into water vapor.
B.Place a tray of water into the freezer and observe it turn to ice.
C.Place a glass of ice outside and observe it melt into water.
D.Place a cold glass of water on the counter and observe it for changes.
C. Place a glass of ice outside and observe it melt into water.
Demetri and Bryan were conducting an investigation in which they placed a container of water in a sunny location and recorded their observations for three days in the chart below.
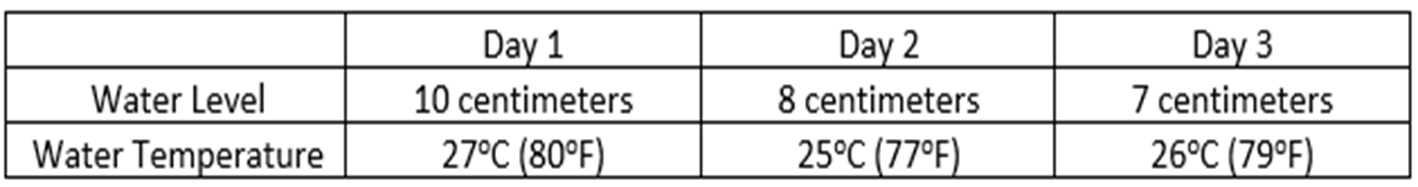
What conclusion could the students make about the water in the container after the three days?
A.The water is condensing.
B.The water is evaporating.
C.The water is boiling.
D.The water is melting.
B. The water is evaporating.
Evelyn and Katie are comparing two samples of matter. The table below shows the properties of each sample.
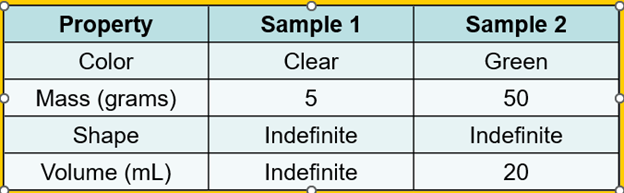
According to the table, what state of matter is Sample 1?
A. Solid
B. Liquid
C. Gas
D. Water
C. Gas
Delilah followed these steps of an investigation:
Step 1 - Collect five objects made of different types of metal.
Step 2 - Place them on a large laboratory table.
Step 3 - Touch each metal object with a magnet and lift slowly.
Step 4 - Record observations.
What is Delilah most likely testing?
A. All types of metal are attracted to magnets.
B. Each magnet can lift the metal object to the same height.
C. Larger magnets can pick up heavier metal objects than smaller magnets can.
D. Heavier metal objects are more attracted to magnets than lighter metal objects are.
A. All types of metal are attracted to magnets.
Brianna and Sindi are studying the affect of surface area on dissolving substances. They want to find out if crushing sugar will make it dissolve faster in water. They get two beakers and fill them both up with 100 mL of water. They add 5g of crushed sugar into one of the beakers as the sample group being tested. The other beaker is the control group. What should they add to the control group beaker and why?
A.5g of crushed sugar; for more data
B.1g of crushed sugar; to compare the difference
C.a 5g uncrushed sugar cube; to compare the difference
D.a 1g uncrushed sugar cube; to compare the difference
C. a 5g uncrushed sugar cube; to compare the difference
One morning, Ryan noticed there were tiny drops of water on the grass as he walked to school. That afternoon, he did not see any drops of water on the grass when he returned home. Which of the following best explains what happened to the drops of water?
A. The heat from the air caused the water drops to boil.
B. The air cooled the water and caused the drops to freeze.
C. The Sun heated the water and caused the drops to evaporate.
D. The energy from the Sun caused the water drops to condense.
C. The Sun heated the water and caused the drops to evaporate.
Jaslene’s class is studying physical properties of solids, liquids, and gases. Her teacher tells the students to make a list of physical properties. Which of the following should NOT be on Jaslene’s list?
A. Color
B. Thermometer
C. Texture
D. Magnetic
B. thermometer
Maria and Tyra were comparing the liquid water in a water balloon to the air in a blown up balloon. Which of the following is most likely a comparison made by Maria and Tyra?
A.Both liquids and gases do NOT have a definite shape, but only gases have a definite mass and volume.
B.Both liquids and gases have a definite volume, but neither have a definite mass or shape.
C.Both liquids and gases have a definite mass, but neither have a definite volume.
D.Both liquids and gases do NOT have a definite shape, but only liquids have a definite mass and volume.
D. Both liquids and gases do NOT have a definite shape, but only liquids have a definite mass and volume.
Jonathan and Sarah have performed an experiment to test the fastest way to separate paperclips and rubber bands. They are not sure if their results are valid. What should they do to check if their results are reliable?
A. average their data
B. change their lab report
C. perform a new experiment
D. repeat the same experiment
D. repeat the same experiment
Jashawn performed an investigation to find out how temperature affects the rate at which sugar dissolves in water. The table shows his results.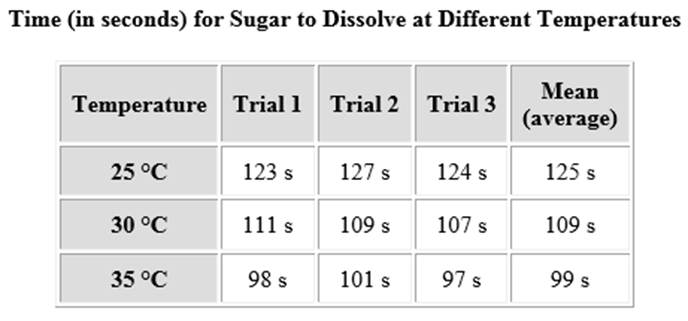
Which of the following is true about the mean (average) values for the time it took the sugar to dissolve?
A. The time needed for the sugar to dissolve decreased as the temperature increased.
B. It is impossible to make any conclusions about Jashawn’s experiment because he did not perform enough trials.
C. The time needed for the sugar to dissolve increased as the temperature increased.
D. The amount of time needed to dissolve the sugar stayed the same no matter what the temperature was.
A. The time needed for the sugar to dissolve decreased as the temperature increased.
Jazmin often boils water in a teakettle. She noticed that sometimes the water boils quickly, and other times it takes a long time to boil. Jahnazia is curious as to why it does not always take the same amount of time for the water to boil. She decides to do a controlled experiment in which she will keep the burner the same temperature for every trial, and she will use the same teakettle for each trial. What data should she collect during her experiment?
A.the amount of water in the kettle and the color of the kettle
B.the amount of water in the kettle and the time it takes for the water to boil
C.the time it takes the water to boil and how the water tastes
D.the temperature of the steam as it leaves the kettle
B. the amount of water in the kettle and the time it takes for the water to boil