What number tells you the number of protons and electrons?
What is the Atomic Number?
the 6 indicators of chemical change are?
What is change in color, odor, light, heat, sound, and bubbles?
Anything that has mass and takes up space
What is matter?
A _____ is a mixture that appears to be a single substance but is composed of two or more substances combined together.
What is the Homogeneous Mixture?
What particle has a positive charge?
What is a proton?
Explain how water could be an example of a physical change.
What is "it can turn easily from ice to water to steam"?
A group of atoms held together by chemical bonds
What are molecule or compound?
A combination of two or more substances that are not chemically combined where you can see the individual substances in the mixture.
What is Heterogeneous Mixture?
How do you figure out the number of neutrons?
What is subtract the Atomic Number from the Atomic Mass?
True or False. Turning peanuts to peanut butter is an chemical change.
What is False, it is creating a mixture?
A type of change where the substance stays the same, but size and shape may change.
What is physical change?
The only way to break down a compound is through this
What is chemical change?
How many electrons fit on the third electron shell?
What is 8?
Which is Not a chemical property? Oxidation, flammability, or density
What is Density?
The smallest particle of matter
What is an atom?
A substance that contains only one type of particle
What is an element?
Draw the particle arrangements for a solid, liquid, and gas.
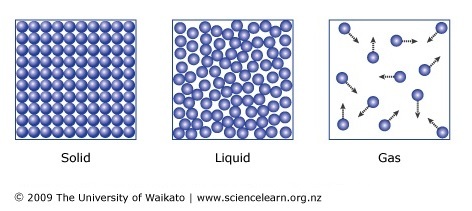
Draw the particle arrangements for a solid, liquid, and gas.
What is 2 and 8?
Type of change where a new substance is created.
What is Chemical Change?
This is a negatively charged subatomic particle
What is a electron?