Matter can be classified as...
(be specific for both)
What is
Pure Substance (element or compound)
or
Mixture (homogeneous or heterogeneous)
observed with senses and determined without changing matter
What is...
A Physical Property
Metals are located....
What is....
On the left side of the Periodic Table
What is the most likely change of state?
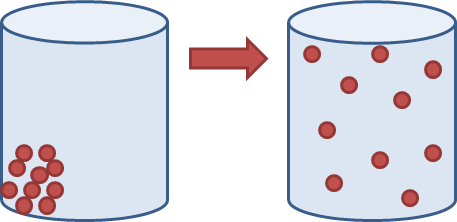
What is...
Solid to Gas
or
Sublimation
As water starts to freeze, the molecules of water...
What is...
decrease in speed
Classify the following matter:
What is...
Pure Substance: Compound
A change in the physical and chemical properties of and object and a new substance is formed
A Chemical Change
Helium (He)
What is...
A Nonmetal
What type of physical change might occur in a substance as energy of particles decreases ?
(must relate to kinetic theoy)
What is...
condensing
or
freezing
or
deposition
Name each state of matter
A. definite shape & definite volume
B. indefinite shape & indefinite volume
C. indefinite shape & definite volume
What are...
A. solid
B. gas
C. liquid
Classify the following matter:
Gold (Au)
What is...
Pure Substance: Element
How are the processes of melting and freezing similar
What is...
They are both processes that physically change a substance.
Cadmium (Cd)
What is...
Metal
What states of matter are involved when nitrogen boils?
What are...
liquid and gas
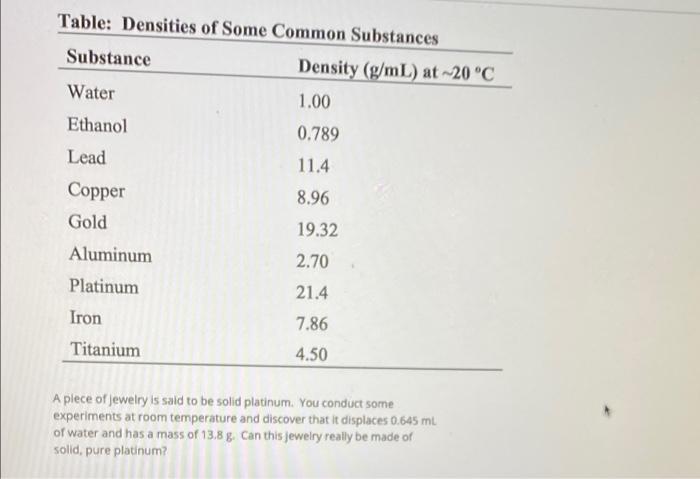
What is the density of a piece of silver that has a mass of 210g and a volume of 20cm3?
(must have correct units)
What is...
10.5 g/cm3
Classify the following matter:
salt water and sand
What is...
Mixture: Heterogeneous
"Flammable" is an example of...
What is...
Chemical property
Silicon (Si)
What is...
A Metalloid

What is...
A. is solid
B. is liquid
C. is gas
The density of water...
What is...
1.0g/mL
Classify the following matter:
Bronze
What is...
Mixture: Homogeneous
(FYI - bronze is a solid mixture of tin and copper)
How does a piece of paper’s "ability to be folded" in half compare to its "ability to burn"?
What is...
"Ability to be folded" is a physical property
"Ability to Burn" is a chemical property
The Periodic Table is made up mostly of...
What are...
Metals
A solid state of matter has the _________________ attraction forces and the ___________________ kinetic energy
What is...
highest (attraction forces)
lowest (kinetic energy)
List from least to greatest density
What is...
Ethanol (0.789) < Water (1.00) < Aluminum (2.70) < Titanium (4.50) < Iron (7.86) < Copper (8.96) < Lead (11.4) < Gold (19.32) < Platinum (21.4)