What is matter?
What do the particles in a solid look like?
They are packed tightly together and cannot move.
What is a mixture?
A substance made by mixing together other substances.
What is Mass?
Mass is how much an object weighs.
Name 3 characteristics of a physical change
●Changes shape or size
●Can dissolve
●Can change states solid, liquid, gas.
●The change is sometimes reversible!
List three ways matter can be described.
Color, shape, texture, size, magnetic.
True or false: The particles in liquids are close, but not packed together. They can move.
True
What is a solution?
A solution is when a mixture is blended into a liquid and cannot be seen.
True or false: When a mixture is created, each ingredient must be weighed and added together.
True: If you are creating a mixture, you must weigh each item. Then you must add those weights together.
●It burns
●Changes color
●A smell forms
●It bubbles
●A substance is transformed into a new substance and looks different from its original state.
True or false: Matter can be described by soft an object is?
Gas particles are spread apart and move quickly.

This homemade lemonade is an example of a solution or a mixture?
Mixture: All the ingredients have been blended and mixed together until the sugar cannot be seen.
Figure out the Mass:
cocoa powder- 50 grams. Water 100 grams
How much would the hot cocoa weigh?
Show your equation.
True or false: Paper cut into smaller pieces is an example of a chemical change. Explain your reasoning.
False: This would be a physical change because the paper did not change into a new substance. It became smaller pieces of the same paper.
What are the three states of matter?
Solid, liquid, gas
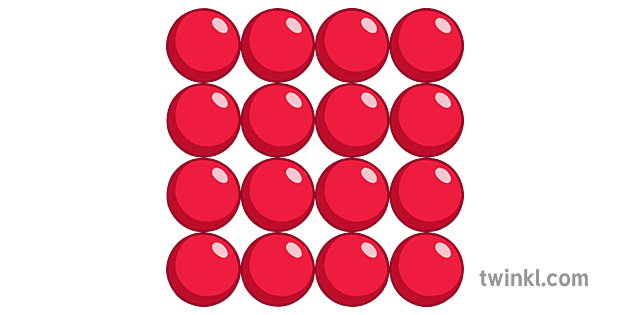
What type of matter do these particles show? Solid, liquid or gas? How do you know?
Solid. The particles are close together and cannot move.
True or false: All mixtures can be taken apart or seperated?
Figure out the mass:
Containers (2)- 5 grams. lemonade powder- 50 grams water-50 grams.
Show your equation.
50+50=100
100-10(container weight)=90.
The cake starts as different ingredients and is turned into a new substance. The cake also creates a new smell.
How can water go through all three states of matter?
Water can start as a liquid. Then if it freezes it can turn into a solid(ice) then if it melts and gets hot enough, the water can Evaporate and turn into gas.
What type of matter do these particles show?
Liquid.
Give an example of a solution.
Answers may vary.
Find the mass.
Timmy is trying to make chocolate milk. He takes out a glass that weighs 20 grams. He adds 70 grams of milk and 30 grams of chocolate milk powder. What is the mass of his mixture? Show your work.
70+30=100
Come up with an example of a physical change and a chemical change that are not in our review packets.
Answers may vary.