In higher temperature particles move_________
What is faster?
Particles vibrate
What are solids?
Liquid to solid
What is freezing?
Ice floats in a cup of liquid water. _________ is more dense.
What is liquid water?
As pressure increases, temperature will _______
What is increase?
Particles are always in ___________
What is motion (moving)?
Have the most amount of kinetic energy
What are gasses?
Solid to liquid
What is melting?
The most dense material is
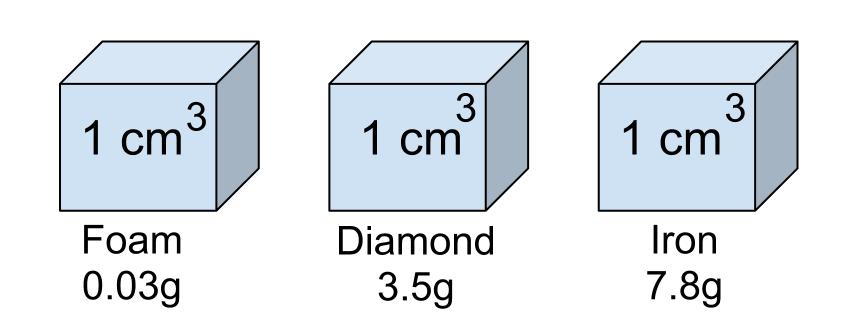
What is iron?
Law that relates Volume and Temperature
What is Charles' Law?
All _____________ is composed of particles
What is matter?
Definite volume but non-fixed shape
What is a liquid?
Gas to liquid
What is condensation?
Objects that float in water are
What is less dense?
The only inverse relationship
What is Boyle's Law?
Larger particles will move ___________ than the smaller particles
What is slower?
Have three laws based on their unique properties
What are gasses?
Solid to gas
What is sublimation?
Density is a measurement of ___________ and __________.
What is mass and volume?
Celsius + 273
What is Kelvin (K)?
Particle motion is ___________ and therefore unpredictable.
What is random?
A state of matter that consists of free-moving ions and electrons
What is plasma?
Gas to solid
What is deposition?
Density is a ___________ property
What is physical?
What are pressure, volume, and temperature?