Chemicals
Polarity + IMF
Reactions
Forensic Principles
Miscellaneous
100
Propane (shown below) contains this kind of bond.
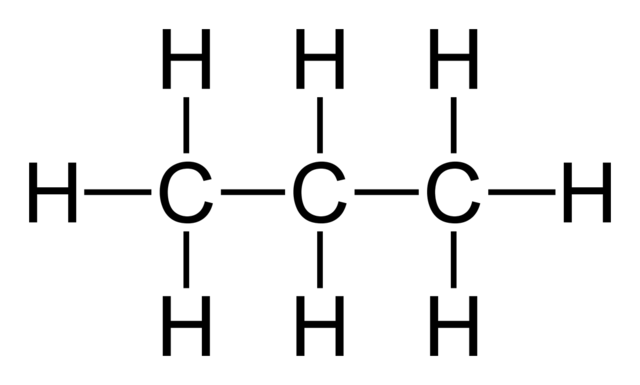
What are PURE COVALENT bonds?
100
Polar or Nonpolar:
H2O

What is POLAR?
100
This is an example of a chemical change:
(A) Gasoline is dangerous because it easily evaporates to fill a given space with flammable vapors.
or
(B) Carbon monoxide kills by bonding irreversibly to red blood cells.
(SELECT ONE)
What is (B) carbon monoxide kills?
100
Unlike eyewitness accounts, most physical evidence handled by forensic scientists is of this type, which requires inference to be related to a crime.
What is CIRCUMSTANTIAL evidence?
100
The type of reaction shown below (of the 5 types):
lead(II) nitrate → lead(II) oxide + nitrogen dioxide + oxygen gas
What is a DECOMPOSITION reaction?
200
The ion in this list that cannot possibly exist:
a) H2+
b) O2+
c) Mg2+
What is H2+?
200
Polar or Nonpolar:
CO2
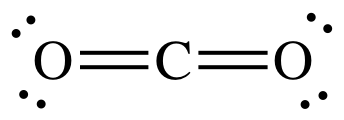
What is NONPOLAR?
200
Balance and type of reaction:
carbon dioxide + carbon → carbon monoxide
What is
CO2 + C → 2CO
(SYNTH rxn)
200
The phenomenon where exaggerated portrayal of forensic science in popular media influences public perception
What is "the CSI effect"?
200
The classic baking soda + vinegar volcano science experiment produces heat when it reacts, making it this kind of chemical reaction.
What is an EXOTHERMIC reaction?
300
The formula for iron(III) bromide.
What is FeBr3?
300
What kind of intermolecular forces?
SF6
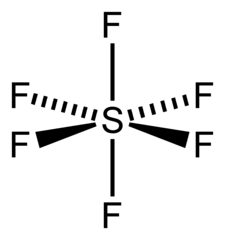
What is induced dipole-induced dipole?
300
Balance and type of reaction:
HNO3 + Al(OH)3 → Al(NO3)3 + H2O
What is
3HNO3 + Al(OH)3 → Al(NO3)3 + 3H2O
(DOUBLE DISPLACEMENT rxn)
300
For this reason, biological samples are often thoroughly dried out prior to storage.
What is "water can catalyze the breakdown of the samples"?
300
According to Le Chatelier's Principle, adding heat to an exothermic reaction will cause the reaction to shift in this direction.
What is shift to the LEFT?
400
The name for P2O5.
What is diphosphorus pentoxide?
400
Formaldehyde (shown below) is an organic molecule. It is most soluble in this kind of liquid. (polar or nonpolar?)

What are POLAR liquids?
400
Balance and type of reaction:
copper + silver(I) nitrate → copper(II) nitrate + silver
_
Nitrate = NO3—
What is
Cu + 2AgNO3 → Cu(NO3)2 + 2Ag
(SINGLE DISPLACEMENT rxn)
400
"The skin under the victim's fingernails is female, which rules out Franklin as a suspect," is an example of this kind of evidence.
What is EXCLUSIVE evidence?
400
Meth (shown below) contains an example of this functional group.

What is an AMINE group? ("Methamphetamine")
500
In The Poisoner's Handbook, HgCl2 is called by its common name, "mercury bichloride." This is the scientific name for HgCl2.
What is mercury(II) chloride?
500
Napthalene, the active ingredient in mothballs (shown below) is most soluble in this kind of liquid. (polar or nonpolar?)

What are NONPOLAR liquids?
500
Balance and type of reaction:
lead(II) nitrate → lead(II) oxide + nitrogen dioxide + oxygen gas
_
Nitrate = NO3—
What is
2Pb(NO3)2 → 2PbO + 4NO2 + O2
(DECOMPOSITION rxn)
500
The last step of evidence procedures is to find a singular common source, called this.
What is INDIVIDUALIZATION?
500
MDMA (shown below) is the active ingredient in ecstasy. It contains two of this functional group.
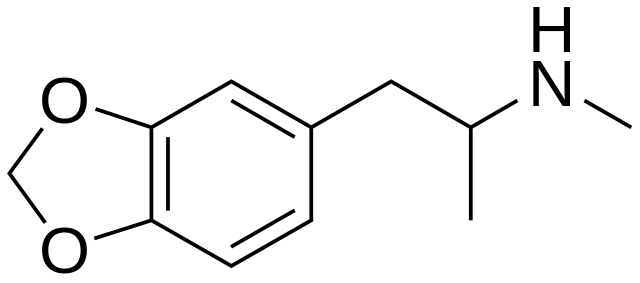
What are two ETHER groups?