What is the definition of the melting point?
The temperature at which a solid begins to to turn into a liquid.
Define a pure substance?
A substance made with one type of material all the way through.
What are the 3 components of a scientific explanation
Claims, evidence, reasoning
What is the blue chemical solution we mixed with aluminum foil? (full name or chemical formula)
Copper Chloride CuCl2
What is the essential question for this unit?
How can I make new stuff from old stuff?
What determines the state of matter?
Temperature
What is the chemical compound for table salt?
NaCl2 (Sodium Chloride)
What is the best way to answer the claim?
Restate the question.
In this reaction, a substance changes color and a burning smell is released. (Chem? or Phys?)
chemical reaction.
What is malleability?
The ability to bend without breaking.
What is the melting point of oxygen?
-218 degrees celsius
Explain in detail how would you check for hardness?
Scratch test. The object that is scratched is the weaker substance.
What should you use to support your reasoning
scientific principles.
What chemicals do you need to make soap?
Oil/ Fat and Sodium Hydroxide
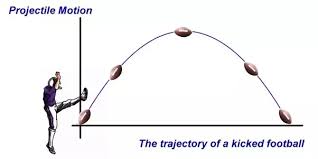
What is projectile motion (definition or drawing)
Draw and label the hierarchy of matter

Define heterogeneous mixture? Give an example.
A heterogeneous is any mixture that is not uniform in composition.
Write the 3 equations for density
D=m/v
M=D* V
D=m/D
A Chemical reaction occurs when a student puts CO2 and H2O together. Write a possible product.
CO2 + H2O ----> ?
What are color change, gas is produced, smell is produced, the reaction is irreversible, temperature change (without cooling or heating,) electrical energy is produced.
What is the universal solvent?
Water
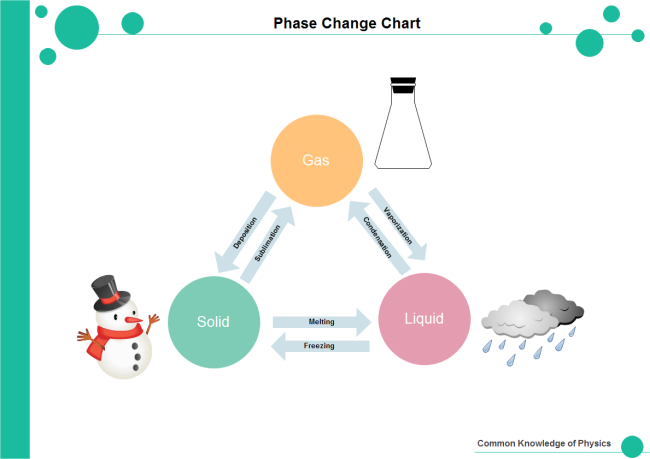
Draw the complete phase change diagram (triangle/pyramid)
Complete the character density for each substance
water
aluminum
soap
fat
water: 1 g/cm3
aluminum: 2.7 g/cm3
soap: 0.84 g/cm3
fat: 0.92 g/cm3
In a chemical reaction what are reactants?
