Therefore, .... is a good way to start which section of a CER?
reasoning
Boiling point is a PP, PC, CC, or CP?
PP
How many oxygen atoms are indicated in this formula?
Al2(CO3)3
9
Where are the protons, neutrons, and electrons. Also, what are their charges?
P(pos) and N(neutral) in the nucleus
E(neg) on the outside
Can all lab chemicals go down the drain?
no
The best tool for measuring liquids...
graduated cylinder
What state of matter has the lowest kinetic energy?
solid
When converting between moles and particles you need to use: Avogadro's number, molar mass, mole ratio, or 22.4 ?
Avogadro's number
changing this will change the element of the atom
protons
where do you put the dependent variable on a graph?
y axis
In experimental design, this is the items/people left under normal conditions.
control group
What is the melting point?
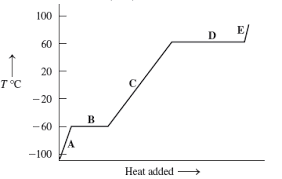
-60
Molar mass of Ca(OH)2
74.09 g/mol
What are TWO differences between nitrogen-14 and nitrogen-15
mass number and number of neutrons
how many electrons in 41Ca2+
18
the _______ variable causes the ________ variable to change.
independent, dependent
Element, compound, or mixture?
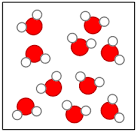
compound
How many moles are in 3.33 g of lithium?
0.48 moles
Give the whole isotope notation
p=9
n=10
e=10
19F-1
How many moles of in 27.8 L of neon gas?
1.24 moles
4700 m is _____ 4.7 km
greater than, less than, equal to
equal to
Evaporating is a PP, PC, CC, or CP?
PC
2Na + CuCl2 --> Cu + 2NaCl
How many moles of Cu are produced by 5.5 grams of Na?
0.12 moles of Cu
Li and Li+ have different numbers of...
electrons
Calculate the avg atomic mass
Isotope A 44.6 amu 92%
Isotope B 45.5 amu 8%
44.672 amu