- 100 — This student correctly states that matter has mass and takes up space.
- "Evan and his friends are working on a group project on matter. Michelle says that matter is anything that has mass. Ricky says that matter must have mass and also be touchable. Evan thinks matter must have mass and also be visible. Leon disagrees and says that matter has both mass and takes up space. Who is correct?"
(Who is Leon?)
100 — Chopping and shaping wood into uniform pieces to build a shelter is this kind of change.
(What is a change in physical property?)
- 100 — This state of matter has particles moving quickly and far apart; one diagram choice would show this.
(What is gas?)
- 100 — A small tank of gas filled many balloons because the gas did this to fill each balloon’s interior.
(What is expand to fill its new containers?)
- 100 — The type of energy that cells convert food into for muscle use is this.
(What is chemical?)
- 200 — The term for a substance’s mass per unit volume; used to identify materials.
(What is density?)
- 200 — Heating sugar until it turns into caramel during baking is this type of change.
(What is a chemical change?)
- 200 — A rain puddle disappearing over a few hours is explained by these processes (general terms).
(What are vaporization and evaporation?)
- 200 — Air stops flowing out of a punctured tire when the internal air reaches this in comparison with outside air.
(What is equal to atmospheric pressure?)
- 200 — The energy type most closely related to temperature is this.
(What is thermal?)
- 300 — The law stating matter is neither created nor destroyed in any change.
(What is the law of conservation of mass?)
- 300 — An air purifier removes dust and allergens from air using this separation method.
(What is filtration?)
- 300 — On Mount Everest water boils at a lower temperature; boiling there won’t reach this standard temperature needed to cook an egg.
(What is 100°C?)
- 300 — Boyle’s law relates pressure and volume at constant temperature; experiments that changed pressure while temperature stayed the same support this law.
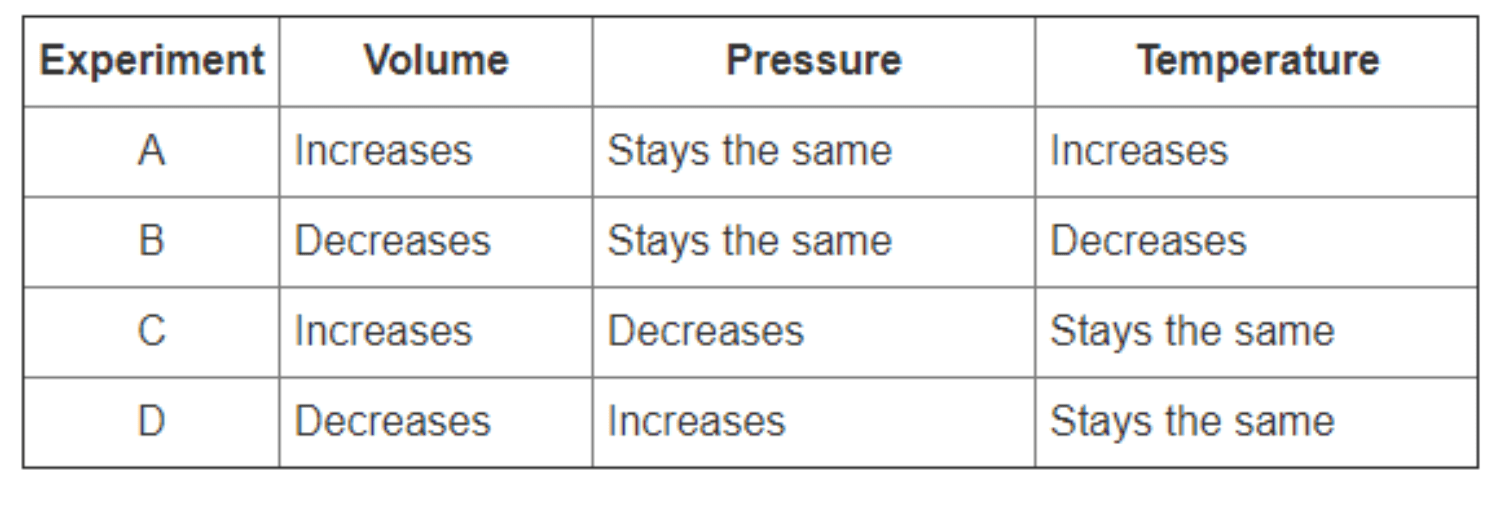
(What are experiments C and D? / or specify which experiments show inverse pressure–volume relationship)
- 300 — Work is done only by the student pushing in the direction of motion; if the box moves east, this student does work.

(Who is Cindi?)
- 400 — The best measurement to compare on Earth and the Moon because it doesn’t change with gravity.
(What is mass?)
- 400 — Crushing aluminum cans without changing the material demonstrates this kind of change.
(What is a change in form or appearance of matter?)
- 400 — A substance that softens and melts over a temperature range, like candle wax, is this type of solid.
(What is an amorphous solid?)
- 400 — A graph of gas volume vs. temperature for Michael was linear; extrapolating, the gas reached 110 mL at this temperature (given the graph’s linear trend).
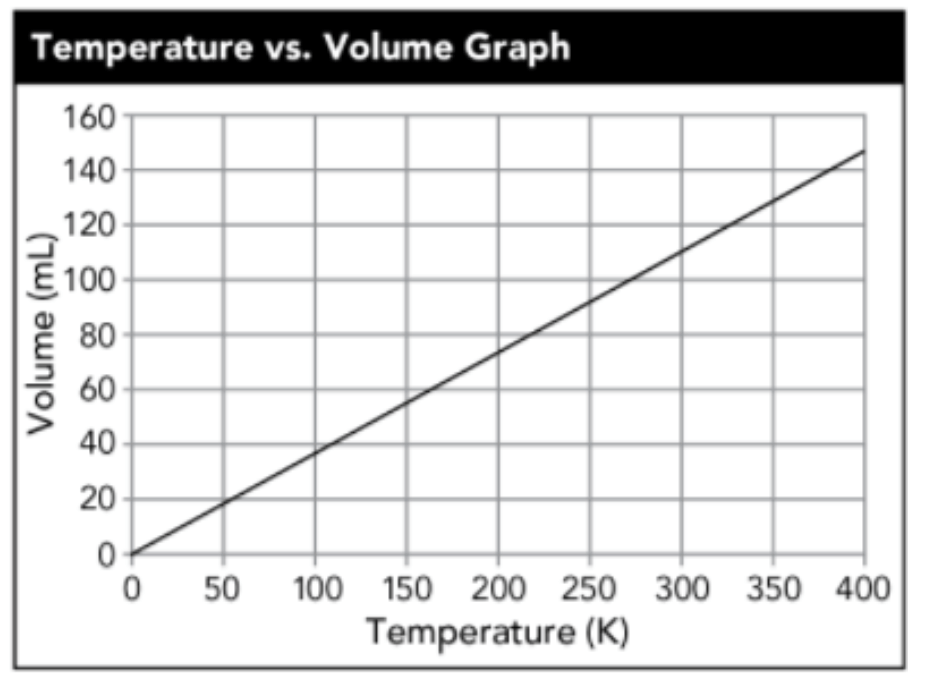
(What is 20 K?)
- 400 — Marissa pushes a 12-kg cart 6 m with a 25 N force; the work done equals this many joules.
(What is 150 N·m (joules)?)
- 500 — The physical property that describes how readily a liquid flows, depending on particle attraction.
(What is viscosity?)
- 500 — Rust forming on metal when exposed to oxygen is an example of this.
(What is a chemical change that produces one or more new substances?)
- 500 — When a fixed-pressure gas’s temperature increases, this happens to its volume.
(What is: the volume will increase?)
- 500 — The property that makes helium-filled balloons rise (lighter than air) is related to this comparison between helium and air.
(What is density? / helium has lower density than air)
- 500 — Lance, a 60-kg runner at 5 m/s, has this kinetic energy.
(What is 750 J?)