These are the subatomic particles in order of charge (negative, neutral, positive)
What are electrons, neutrons, and protons?
representation of an atom or molecule using only the element symbol and the valence electron arrangement
What is a lewis dot diagram?
What is a lewis structure?
What is an electron dot diagram?
This element is a liquid at STP.
What is Mercury or Bromine?
An aqueous solution is classified as this type of mixture.
What is homogeneous?
The diagram below shows different radioactive emissions being fired through a magnetic field.
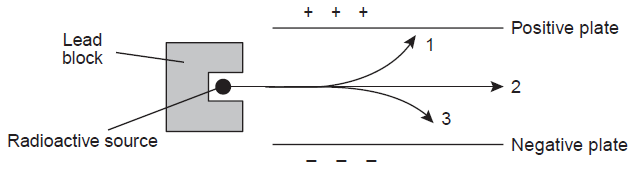
This radioactive particle could represent emanation #3.
What is.. an alpha particle? What is a positron? What is a proton?
This classification of matter consists of two or more different elements chemically combined in a fixed proportion.
What is a compound?
As an atom in the ground state changes to an atom in an excited state, the atom
What is absorbs energy?
the group of elements on the periodic table that do not follow the typical reactivity and valence electron trends
what is transition metals?
what is groups 3-12?
This type of reaction will cause the temperature of thes surroundings to increase.
What is exothermic reaction?
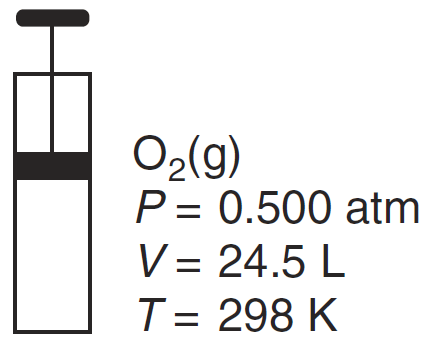
These changes in volume and temperature of the gas in the cylinder would decrease the frequency of collisions between the O2(g) molecules.
What is.. increase the volume and decrease the temperature?
What is 24.5 L > V and T < 298 K?
medical applications such as treating cancer and thyroid disorders, carbon dating, geological dating.
What are uses of radioisotopes?
The experiment responsible for discovering that all atoms contain negative particles embedded within.
What is the Cathode Ray Tube experiment?
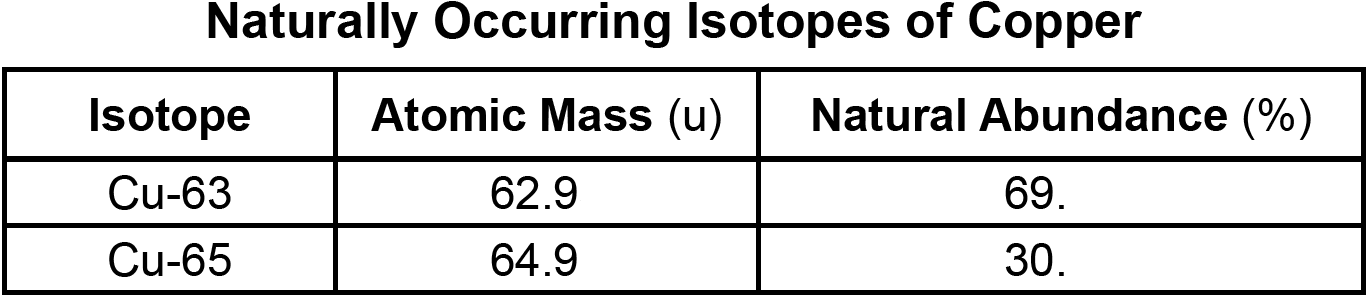 Based on the data, the atomic mass of copper to the nearest hundredth.
Based on the data, the atomic mass of copper to the nearest hundredth.
What is 63.52?
An electron in this shell of strontium in the ground state has the most energy.
What is the 5th shell?
A mixture of sand and water are able to be separated by filtration due to this.
What is the sand is insoluble in water?
Mercury, water (1.000g/mL), carbon tetrachloride (1.595g/mL), and bromine are placed in a graduated cylinder and layers formed. Identity the order of layers from top to bottom.
What is... water, carbon tetrachloride, bromine and mercury?
This is the balanced nuclear equation for the natural transmutation of Technetium-99.
What is...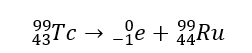
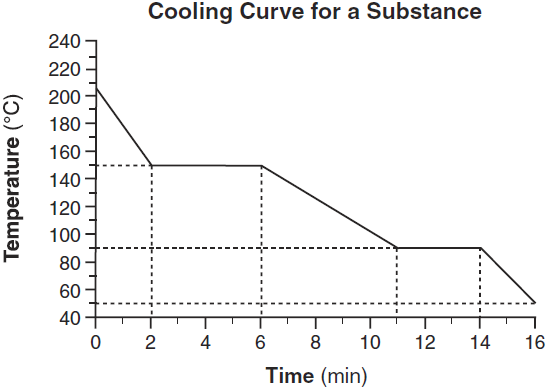 The melting point of the substance.
The melting point of the substance.
What is... 90°C?
The gold foil experiment led to the conclusion that each atom in the foil was composed mostly of empty space. This specific observation determined this fact.
What is most alpha particles passed through the foil undeflected?
This group of elements are the most reactive metals and why.
What is the alkali metals?
What is they lose their valence electron the most easily?
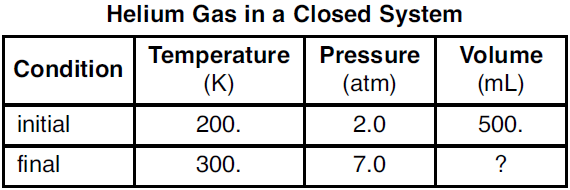 The final volume of the helium gas.
The final volume of the helium gas.
What is 214 mL?
A sample of helium gas, He(g), is placed in a rigid cylinder sealed with a movable piston under constant temperature. Compare the number of helium atoms in the cylinder at volume of 2.5 mL and 1.0 atm to the number of helium atoms in the cylinder when the volume is changed to 1.25 mL and 2.0 atm.
What is.. the number of atoms remains the same.
This mass of an original 5.60-gram sample of iron-53 that remains unchanged after 25.53 minutes.
What is 0.70 grams?
Data for the concentration of CO2(g) in the atmosphere from 1960 to 2000 is shown in the table below.
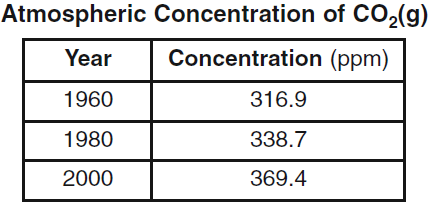
The atmosphere is classified as a mixture for this reason.
What is.. the concentration of carbon dioxide can vary?
In what order should these historical developments leading to the modern model of the atom be placed. (From oldest to youngest)
Most of the atom is empty space
The atom is a hard sphere
Electrons exist in orbitals outside the nucleus
What is... the atom is a hard sphere, most of the atom is empty space, electrons exist in orbitals outside the nucleus?
The reason that Fluorine has a greater electronegativity than Carbon.
What is... a stronger nuclear charge?
The below diagrams show three samples of matter at STP.
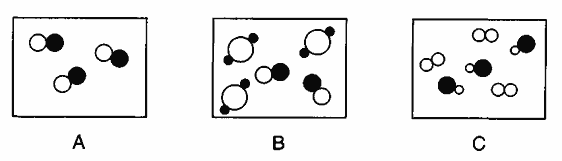
The average kinetic energy of sample B equals the average kinetic energy of sample C for this reason.
What is... the samples are at the same temperature?
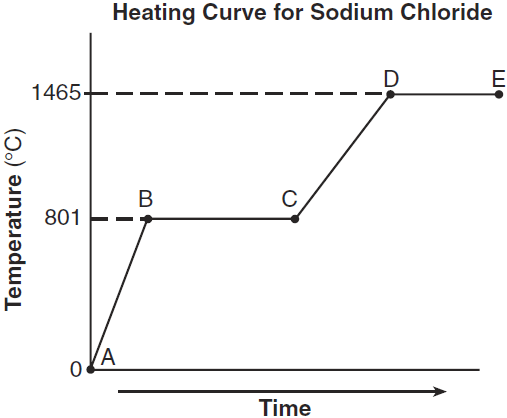
The temperature range in which the entire sample is a liquid.
What is... 801°C to 1465°C?
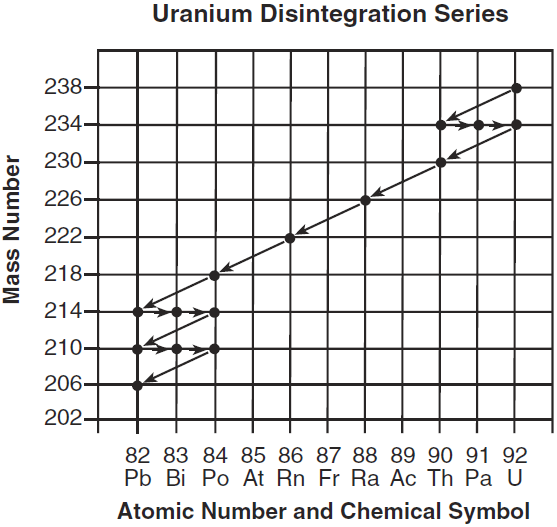
The reason the decay series of U-238 ends with Pb-206.
What is.. the nucleus if Pb-206 is stable?
The below curve represents the heating ammonia starting as liquid.
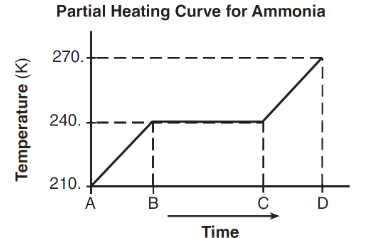
This DRAWING represents the phases of the particles of ammonia during CD.