What would be the molar mass of compound R2D3 if the molar mass of R is 11.99 g/mol and the molar mass of D is 20.75 g/mol?
86.23 g/mol
Find molar mass of the compound just like you would any other.
11.99(2) + 20.75(3) = 86.23 g/mol
Another example: molar mass of H2O is 1.01(2) + 16.00 = 18.02 g/mol
4 Fe + 3 O2 ->2 Fe2O3
How many moles of oxygen would be required to react with 7.5 moles of iron?
5.63 mol O2
Mole to mole = 1 step, just use your mole to mole ratio (mole over mole fraction from balanced equation)
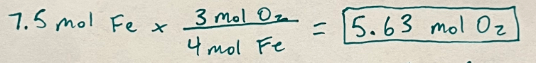
What are the three ways to increase the rate of solubility?
Shake, break, and bake
(stir, smaller pieces, and higher temperature)
A student used 2 M stock solution of HCl to make a 5 L of a solution with a molarity of 0.30 M. What volume of the stock solution did the student use?
HINT: any time you have 2 volumes or 2 molarities, you will use the dilution formula
Answer: 0.75 L
The dilution formula is M1V1 = M2V2
M1 and V1 are the molarity and volume of the stock solution (the original)
M2 and V2 are the molarity and volume for the new solution that you are making
In this question it asked for the volume of the STOCK solution so you are solving for V1
If you have 70.00 g of Z2, how many moles of Z2 do you have? The made up element Z has a molar mass of 40.50 g/mol.
0.86 mol Z2
Start with 70.00 g of Z2. Put g of Z2 on the bottom (40.50 x 2) and 1 mol on top.
K2SO4 + 2 HBr -> H2SO4 + 2 KBr
Observe the reaction above. How many grams of KBr will be produced when 15 g of HBr is reacted with excess K2SO4?
Grams to grams is 3 steps. (the excess chemical DOES NOT MATTER)
22.06 g KBr
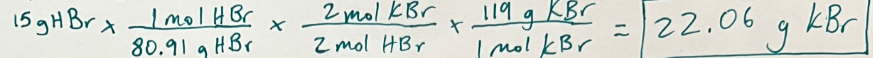
What is the molarity of a solution made of 12 moles of NaCl in enough water to make a 4 L solution?
3 M
Molarity = moles / liters = 12 mol / 4 L
A student used 20.10 mL of a stock solution of HCl to make 100 mL of a solution with a molarity of 0.25 M. What is the concentration of the stock solution?
HINT: if both volumes are mL, then you don't have to convert to L. You will get the same answer.
Answer: 1.24 M
The dilution formula is M1V1 = M2V2
M1 and V1 are the molarity and volume of the stock solution (the original)
M2 and V2 are the molarity and volume for the new solution that you are making
In this question you are finding the concentration of the stock solution so you are solving for M1
Sarah has an unknown compound made of new elements. In the lab, she determined that the unknown compound was 15.8% element X and 84.2% element Y. Calculate the empirical formula using the molar masses given below:
X molar mass = 24.02 g/mol
Y molar mass = 64.14 g/mol
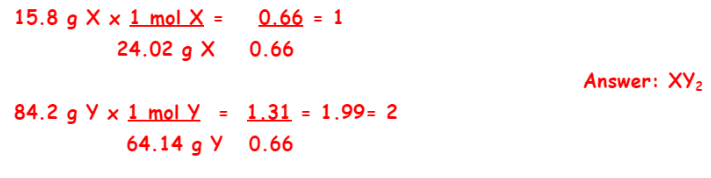
A scientist reacts sodium and chlorine together to make sodium chloride. After the reaction is complete, there is some sodium metal left over in the beaker.
Sodium metal is the _______________ reactant and chlorine is the _________________ reactant.
Sodium metal is the EXCESS reactant and chlorine is the LIMITING reactant
(excess=extra)
What is the molarity of a solution made of 32.04 g of BeF2 dissolved in enough water to make 0.90 L of solution?
Answer : 0.76 M
Molarity = moles / liters.
We have liters but we don't have moles. We need to convert the grams to moles. Start with 32.04 g, then put g on the bottom (molar mass of BeF2) and 1 mol on top to convert to moles.
Molar mass BeF2: 9.01 + 19.00(2) = 47.01 g
32.04 x 1 / 47.01 g = 0.68 moles
Now you can do moles divided by liters
0.68 moles / 0.90 L = 0.76 M
What is the MOLECULAR formula of a substance with mass of 64.20 g/mol and an empirical formula of CH4?
Hint: molecular formula is UNSIMPLIFIED
Answer: C4H16
The question states that the empirical formula is CH4 and asks you to find the unsimplified version (molecular formula such as C2H8 or C3H12). To find the unsimplified version, take the mass given 64.20, and divide by the mass of the empirical formula (CH4 has a mass of 16.05). You should get 4. This means that you need to multiply all the subscripts in CH4 by 4. This will give you C4H16 as your answer.
A student runs the following reaction in the lab:
K2SO4 + 2 HBr -> H2SO4 + 2 KBr
The student starts the reaction by combining 3 moles of K2SO4 and 3 moles of HBr each. What is the excess reactant?
Answer: K2SO4
Your choices are K2SO4 and HBr because those are your two reactants. Figure out how many moles of product you can make with each reactant (pick 1 product to focus on)
Convert 3 moles of K2SO4 to moles of H2SO4 (1 step stoich problem, 1 mol K2SO4 on bottom and 1 mol H2SO4 on top) = 3 moles H2SO4
Convert 3 moles of HBr to moles of H2SO4 (1 step stoich problem, 2 mol HBr on bottom and 1 mol H2SO4 on top) = 1.33 moles H2SO4
The reactant that gives you the BIGGER answer is the EXCESS reactant
The reactant that gives you the SMALLER answer is the LIMITING reactant
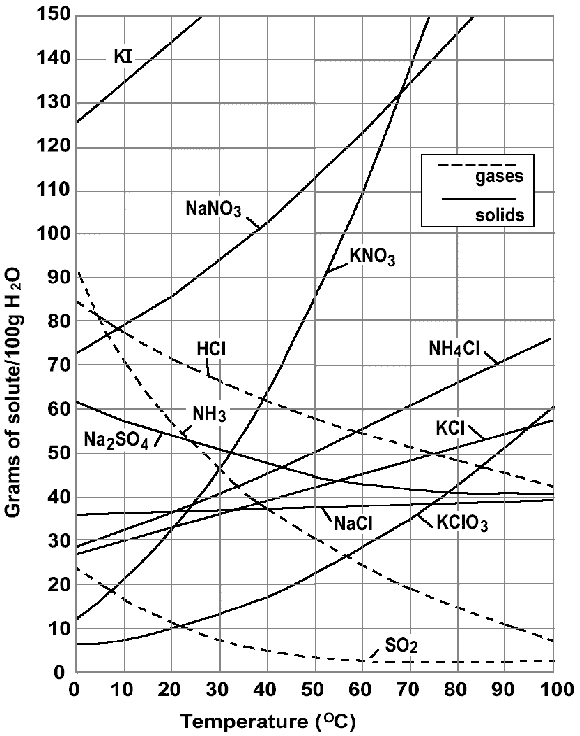
When 60 g of ammonium chloride (NH4Cl) are dissolved in 100 g of water at 90 degrees Celsius, what kind of solution do you have: saturated, unsaturated or supersaturated?
Unsaturated
How many molecules of H2O are found in a 175 g sample of H2O?
Hint: this is a 2 step problem
Grams to molecules = 2 steps. First convert grams to moles (grams on bottom 1 mol on top). Then convert moles to molecules (1 mol on the bottom and avogadro's number on top)
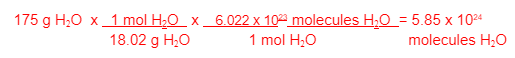
1. Both ___________ and ____________ compounds undergo solvation.
2. Only ____________ compounds undergo dissociation when placed in water.
3. Is CaF2 ionic or covalent?
4. Is C2H6O2 ionic or covalent?
1. Both IONIC and COVALENT compounds undergo solvation.
2. Only IONIC compounds undergo dissociation when placed in water.
3. CaF2 is IONIC because it is made of 1 metal and 1 nonmetal (1 element on the left of the staircase and 1 element on the right of the staircase)
4. C2H6O2 is COVALENT because C, H, and O are ALL nonmetals
Which of these would have the highest boiling point?
a) 4 M CH4
b) 4 M BeF2
c) 4 M NaCl
d) 4 M AlCl3
e) pure water
d) 4 M AlCl3
More solute = more boiling point elevation
The one with the highest mass will make the boiling point elevate the most