This form of matter has a fixed composition and can be broken down chemically.
What is a compound?
Which sample of water has particles with the greatest average kinetic energy?
A) 50.g at 10°C
B) 80. g at 25°C
C) 5.0 g at 60°C
D) 200.g at 0°C
What is C, 5.0g at 60°C?
At STP, this gas sample contains the same number of molecules as 1.5 L of O₂(g).
A) 0.75 L N₂
B) 3.0 L H₂
C) 1.5 L CO₂
D) 2.0 L Ne
What is C, 1.5 L of CO2
What are ions?
The ability of an atom to attract electrons.
What is electronegativity?
Given the equation representing a reaction: I₂ → I + I. What changes happen to the bond and energy?
Bonds are broken and energy is absorbed.
This property of a substance can be observed without changing the identity of the substance.
What is a physical property?
Convert 428oC to Kelvin.
What is 701 K?
Draw the relationship between volume and temperature.
[Direct Relationship]
Name one conclusion about the structure of the atom as a result of the gold foil experiment.
The atom has a small, positive, dense nucleus
OR
The atom is mostly empty space
Different forms of the same element. They have different structures and different properties.
What are allotropes?
This substance has metallic bonding.
A) Cu
B) Br2
C) CO
D) H₂O
What is A), Cu or Copper.
A student separates sand from saltwater by pouring the mixture through filter paper. This laboratory technique separates substances based on differences in particle size.
What is filtration?
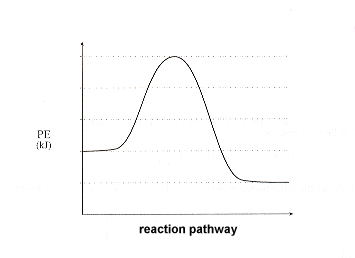 Label the heat of reaction with a double headed arrow.
Label the heat of reaction with a double headed arrow.
Arrow between the products and reactants.
The kinetic molecular theory of gases includes several statements that explains how ideal gases behave. Name one of these statements.
1) Gases have negligible volumes
2) Gases move in constant, random, straight-line motion
3) Gases do not experience intermolecular forces
4) Gases move proportional to temperature
5)All collisions are elastic (bouncy) and no energy is lost
The net charge of an atom that has 13 protons, 10 electrons, and 14 neutrons.
What is +3?
As atomic number increases across Period 3, atomic radius generally does this.
What is decrease.
The Lewis Dot Diagram for NH3.
[Teacher Check, pyramidal shape]
In this sample, the ratio of components can vary:
A) KBr(s)
B) CO₂(g)
C) NH₃(g)
D) NaCl (aq)
What is D, NaCl(aq)?
A student holds a glass of water at 55°C in a room where the air temperature is 20°C, and the student’s hand temperature is 37°C. Identify two directions of heat flow that occur in this system.
What is from the water to the hand, and from the water to the air?
Convert 152 kPa to atmospheres.
What is 1.50 atm?
Compare the energy of an electron in the 1st shell vs the 3rd shell.
This element is a metalloid:
A) Al
B) Si
C) Na
D) S
What is B), Si?
The bond polarity and molecular polarity of CO2.
What is polar bond and non-polar molecule?
Explain, in terms of particle distribution, why NaCl(aq) is a homogenous mixture?
The particles are distributed evenly or uniformly.
This amount of heat is required to completely vaporize 25.0 g of water at 100°C.
What is 56,500 J ?
A sealed container holds helium gas at 530. K and 104 kPa. If the temperature decreases to 450. K, calculate the new pressure.
What is 88.3 kPa?
Write an electron configuration for K in the excited state.
2-8-7-2
[other answers are possible and subject to approval]
It's why electronegativity decreases down a group.
What is increased principle energy levels or shells.
The ions in a solid cannot move, but can move when dissolved.