There are this many significant figures in the number 0.0030
What is 2?
This family includes highly reactive non-metals with 7 valence electrons such as I, Br, and F
What is the halogens?
This type of bond forms between 2 nonmetals, where both atoms share their valence electrons.
What is a covalent bond?
The formula of the compound Magnesium Chloride
What is MgCl2?
This is the balanced equation for the combustion of methane (CH4).
What is
1CH4 + 2O2 ➜ 1CO2 + 2H2O
This is the number of neutrons in Selenium-76
What is 42?
This is the neutral element represented by the electron configuration
[Ne] 3s23p3
What is Phosphorous?
This is the type of bond in the compound BaF2
What is ionic?
The name of the compound Fe3(PO4)2
What is Iron(II) Phosphate?
Fe2(SO4)3 + KOH → K2SO4 + Fe(OH)3
This is how many moles of K2SO4 would be produced from 3 moles of KOH
What is 1.5 mol K2SO4?
The Density of a substance with a volume of 4.5 mL and a mass of 23 g
What is 5.1 g/mL?
This rule states that no two electrons in an atom can have the same 4 quantum numbers.
What is Pauli Exclusion Principle
This is the Lewis Dot Structure of the compound CH4
What is

There are this many grams in 4.20 moles of TeF4
What is 855 grams
Fe2(SO4)3 + KOH → K2SO4 + Fe(OH)3
This many grams of Fe2(SO4)3 would be required to produce 89.2 g of Fe(OH)3
What is 167 g Fe2(SO4)3
This scientist is responsible for the Gold Foil Experiment, which led to the discovery of the nucleus.
Who is Ernest Rutherford?
The elements Al ,Cl, and K arranged in order of increasing electronegativity.
What is K, Al, Cl?
This is the Lewis Dot Structure and Molecular Geometry of CO2
What is Linear?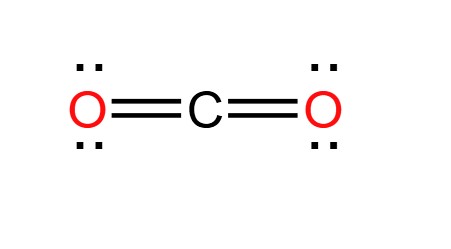
This is the percent of O in the compound glucose, C6H12O6
What is 53.28%
Fe2(SO4)3 + KOH → K2SO4 + Fe(OH)3
This is the % yield of a reaction where 58 g of KOH produced 31.4 g of Fe(OH)3
What is 85.27%?
The average atomic mass of Gallium based on the following data:
There are 2 isotopes of Gallium.
Ga-69 has a mass of 68.9256 amu and an abundance of 60.11%. Ga-71 has a mass of 70.9247 amu and an abundance of 39.89%.
What is 69.7230 amu?
The frequency if a photon has 4.00 x 10-19 J of energy.
What is 6.04 x 1014 Hz?
This is the correct resonance structures for the compound CO3-2
What is
This is the molecular formula of a compound that is composed of 66.67% C, 3.73% H, and 29.60% O and has a molecular weight of 216.192 g/mol.
What is C12H8O4?
Fe2(SO4)3 + KOH → K2SO4 + Fe(OH)3
This is the limiting reactant in a reaction between 162 g of Fe2(SO4)3 and 48.1 g of KOH.
What is KOH?