This is the second element listed on the periodic table of elements
What is helium?
The atomic nucleus is composed of these two particles.
What are protons and neutrons?
These are the units for molecular weight
What are grams per mole?
Or, with a slightly irritated look from the TA:
What are Atomic Mass Units?
This scale ranks the hardness of minerals, with soft minerals being 1, and very hard minerals being 10
What is the Moh's hardness scale?
This is the name of your TA.
Bonus 100 points: What is his email?
Who is Nabila Nizam?
Bonus: wnelson@hawaii.edu
This element has 79 protons
What is gold?
Atoms that have lost or gained electrons are called this
What are ions?
This is how many items in a mole
What is avagadro's number?
Or what is 6.022*1023 atoms per mole?
This mineral is clear, scratches glass, and exhibits conchoidal fracture

What is quartz?
This many radians are in 180 degrees?
After oxygen, this is the most abundant element in the crust of the Earth.
What is Silicon?
The quantity of these particles determines an element's atomic number?
What are protons?
This is the molecular weight of CaCO3 (can be approximate) in g/mol
BONUS 100 points: What is the name of this mineral?
What is around 100 g/mol?
Ca= 40.08
C = 12.01
O = 16 (*3) = 48
= 100.06
Bonus: Calcite
This mineral is often clear, you can scratch with your fingernail, and has a white streak.

What is gypsum?
This is what you are testing for when you spray a rock with acid.
What is calcite?
Secret bonus 100: What are carbonates?
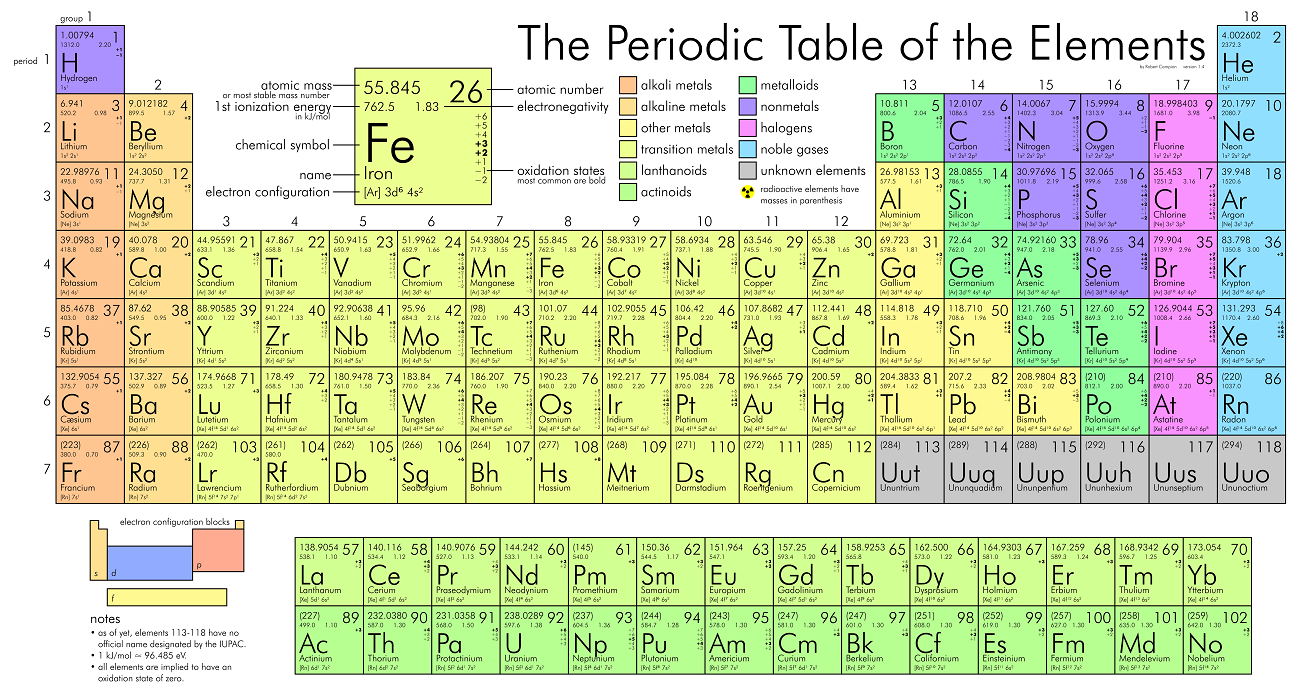
Elements who share this location on the periodic table tend to have the same properties.
Choose from:
period or group
partial credit (300 points) choose from:
Row or column
Partial:
What is a column?
These particles determine the bonding characteristics of an atom, and lie in the outermost layer of the electron cloud?
What are valence electrons?
These are the three types of bonds that involve valence electrons, and their characteristics.
What are ionic bonds (transfer of electrons), covalent bonds (sharing electrons), and Metallic bonds (sharing electrons which are allowed to move freely)?
Suppose homework is worth 20% of your grade, quizzes are worth 30% of your grade, and exams are worth 50% of your grade.
This is your grade if you scored and average of 74% on homework, 95% on quizzes, and 81% on exams.
What is 83.8%?
.2*74+.3*95+.5*81=83.8
Choose from:
Ca, K, Mg, Na
What are Ca and Mg?
These two elements are in the same column (or group)
Both have 2 electrons in their outer subshell, making them prone to electron donation.
This element has the complete electron configuration:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3
What is arsenic (As)?
This is the mass (in grams) of one mole of carbon atoms
Extra info:
Carbon has an atomic mass of about 12 g/mol
Avagadro's number= 6.022*2023
Plank's constant= 6.626*10-34 m^2 kg/s
What is 12 grams?
12 g/mol *1 mol= 12 g
This is how sunglasses dim light passing through them.
Bonus -100 points: "What is making the light darker?"
Bonus 100 points: Describe how this works (briefly)
What is polarization?
Bonus: Sunglasses basically filter out light with E fields oscillating in all directions but one, preserving the whole image, but reducing the overall intensity of the light going through them.
Or anything that basically presents this idea:
