Why are WHMIS symbols important?
Allows you to know how to handle, store and dispose materials.
What can Matter be classified as?
Pure Substance VS Mixture
What makes a solution concentrated?
Large amounts of solute inside a solvent.
What two parts make a solution? Provide an example.
Solute - the substance being dissolved
Example: sugar, salt
Solvent - the substance that does the dissolving ( usually a liquid)
Example: Water
What does WHMIS stand for?
Workplace Hazardous Materials Information System
Provide two examples of a mixture
Answers may vary
Which of the following is the most concentrated?

Most to the right
What does solubility mean?
The maximum amount of solute that can be dissolved in a specific amount of solvent at a given temperature.
What is this WHMIS symbol? What does it mean?

Biohazardous - Risk to human health and enviornment.
What is a pure substance? Provide an example.
Anything that is made up of one type of matter. Example: Gold, Sugar, Salt, Aluminum
How do you write out the concentration of this solution?
5g of salt
15 mL of water
5g / 15 mL
What does saturated and unsaturated mean?
Saturated - A solution in which no more solute can dissolve at a given temperature.
Unsaturated - More solute can be dissolved in the solution at a given temperature.
What is this WHMIS symbol? What does it mean and where would you see it?
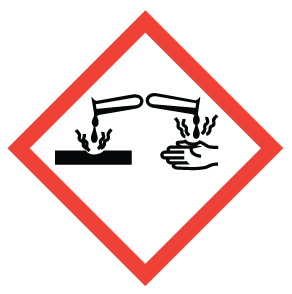
Corrosion - Corrosion to eyes, skin and/or metal. Harm or destroy skin layer. Can be found on strong acids and bases. Example: Sulfuric Acid and Bleach
What is does it mean by homogenous and heterogenous? Provide examples of each.
Homogenous - Even distribution throughout, different layer can not be seen. Example: Apple Juice
Heterogenous - Different layers or parts can be easily spotted. Example: Salad
Which solution is more concentrated:
1. 60 g / 100 mL
2. 30 g/ 100 mL
60g / 100mL
List from most to least soluble. 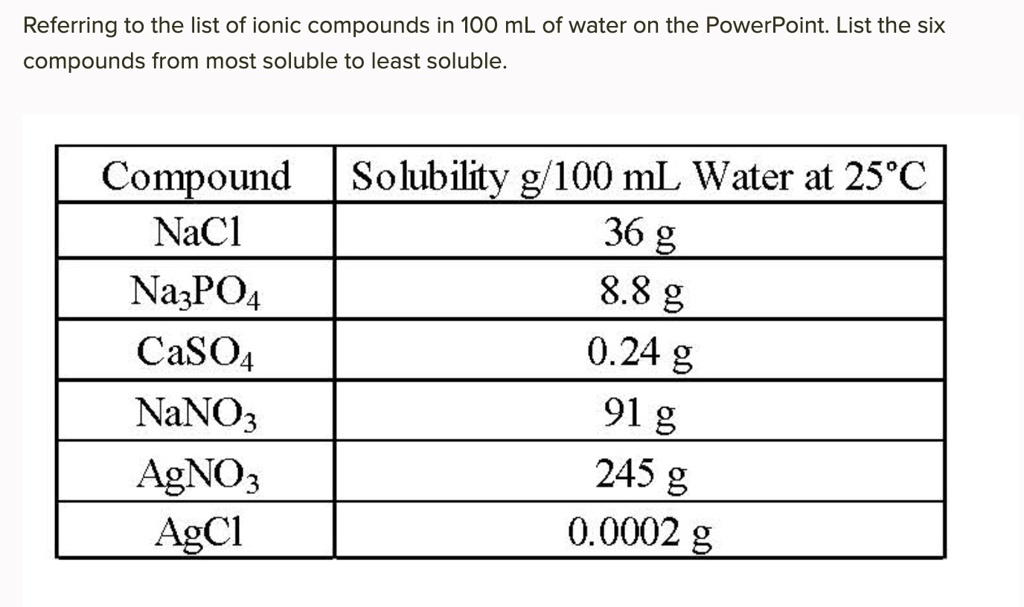
AgNO3 > NaNO3 > NaCl > Na3PO4 > CaSO4 > AgCl
Pick at least 3 WHMIS symbols to describe. Name of symbol, meaning and example.

Answers will Vary
Where would "mechanical mixtures" and "solutions" fit under the classification? Provide examples.
Mechanical Mixture - Heterogenous
Solution - Homogenous
Examples will vary
Which solution is more concentrated?
1. 300 mL of water with 60 g of sugar
2. 150 mL of water with 35 g of sugar
150 mL of water with 35 g of sugar
The saturation point of a solution measures the solubility of the solution. When a solution reaches its saturation point, it becomes saturated. Before a solution meets its saturation point, it is considered unsaturated and more solute can be dissolved.