The part of the solution that does the dissolving (water, vinegar)
What is a solvent?
The property that we measure with a balance scale.
What is mass?
What does it mean when you see sugar in the bottom of a pan of boiling sugar solution?
What is the solution is supersaturated?
A tool used to separate solids from a solution
What is a filter?
Sodium Chloride (NaCl)
What is salt?
A pure material.
What is a substance?
The property that allows heat and electricity to move easily.
What is conductivity?
Fireworks are evidence of what type of change?

What is a chemical change?
What do Kool-Aid powder, salt, citric acid and baking soda have in common?
What are solutes?
Evidence of a chemical change. Name 3.
What is color change, heat, gas production, sound, and production of a precipitate?
A solid that forms after a chemical reaction.
what is a precipitate?
What physical properties of matter can be observed with our senses and/or measured with a tool? Name 3.
What is solubility, conductivity, magnetism, density, temperature, mass, color, volume, texture?
Pouring soda water in a cup is evidence of what kind of change.

What is a physical change?
The ability of a substance to mix into a liquid
What is solubility?
This Law states that the amount of matter does not change during a chemical change.
What is the Law of Conservation of Matter?
The process in which two or more substances combine to make one or more new substances.
What is a chemical reaction?
This state has particles that move very quickly and are far apart
What is a gas?
How does heat effect a solution of sugar and water?
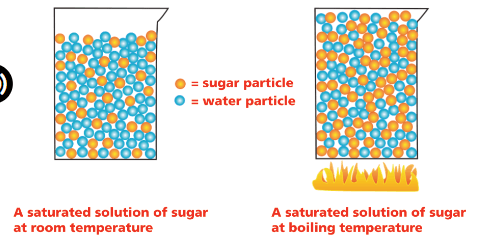
What is causes it to dissolve more solute(sugar)?
When a cup of lemonade tastes sweeter than another cup of lemonade we say that it is
What is more concentrated?
The amount of space an object takes up
What is volume?
A solution that contains the maximum amount of solute.
What is a saturated solution?
The property we explore with sink and float.
What is density?
Describe how the pot of orange juice (solution) changes after being heated.
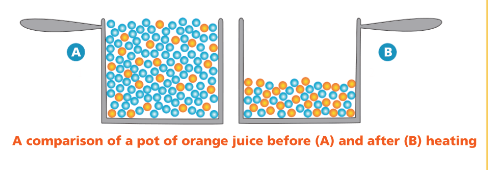
What is the number of water molecules decreases and the solution becomes concentrated?
Adding more solvent to a mixture makes the mixture
What is more diluted?
The effect concentration has on density.
What is the more concentrated matter is, the more dense it is?