Democritus went against Aristotle's blended soup idea of earth, wind, fire and water. Instead, he said that everything in the world is made of tiny "atomos" particles. "Atomos" in Greek means __________.
Indivisible
Who first arranged cards in order of increasing atomic mass and grouped them into families, but was also bold enough the predict the existence of undiscovered elements?
Dmitri Mendeleev
The number of which type of subatomic particle is solely responsible for determining the identity of the element.
protons
When an atom has another version that is heavier or lighter because of a different number neutrons, those versions of that atom are called ________________?
Isotopes
Which group on the periodic table represents a family of elements that are all gases?
noble gases or group 18
Rutherford proved through the gold foil experiment that most of an atom is actually made up of __________ ___________.
empty space
The last (very right) group on the periodic table is called the ______________.
noble gases
Write the nuclear symbol for an atom with 9 protons, 10 neutrons and 9 electrons.
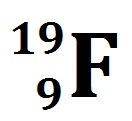
Write the hyphen notation of what is most likely the most abundant isotope of the period three alkali metal?
Na-23 or sodium-23
Which metalloid shares properties of both metals and non-metals, but was incredibly important in making computer chips because it only conducts electricity "a little bit"?
silicon
The following is called JJ Thomson's "__________" model.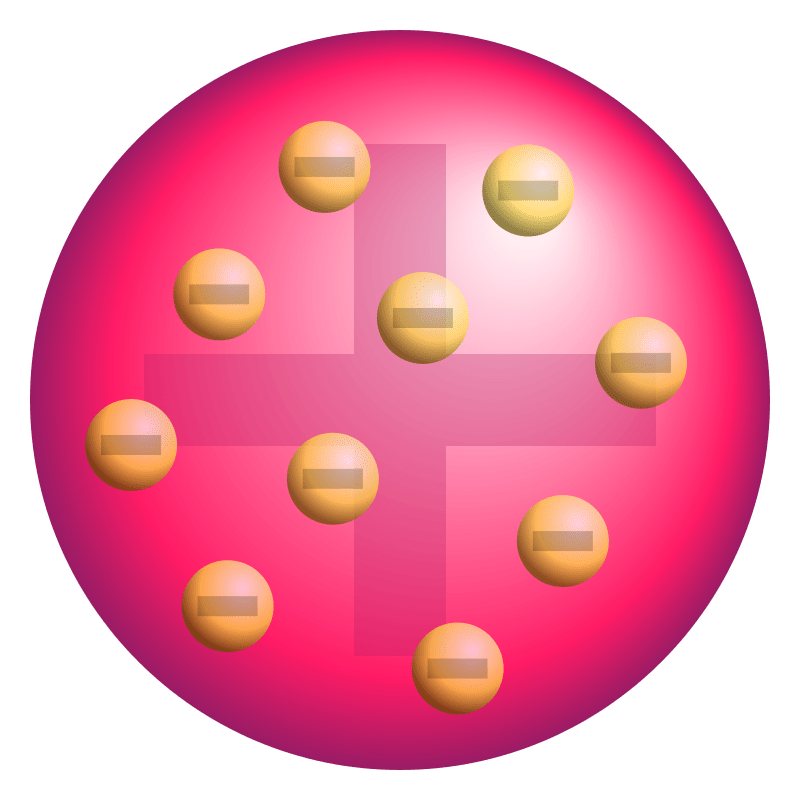
Plum Pudding
Horizontal rows on the periodic table are called ________________.
Periods
When an atom loses electrons and becomes ____________ charged, it turns into what chemists call a(n) _____________.
positively, cation
Calculate (rounding to the hundredths place) the average atomic mass of the following element:
Cu-63 62.93 amu AND 69.17% abundance
Cu-65 64.93 amu AND 30.83% abundance
63.55 amu
What two groups of atoms are horizontal and would extend the length of the periodic table if they were inserted where they belong?
lanthanide series and actinide series
Ernest Rutherford is credited for discovering that most of the positively charged mass of an atom is concentrated in a tiny space at the center of the atom, called the ___________________.
Nucleus
Name an Alkaline Earth Metal in period three.
What is the name of the element AND the number of protons for an anion with a 3- charge, which contains 10 electrons?
Nitrogen, 7 protons
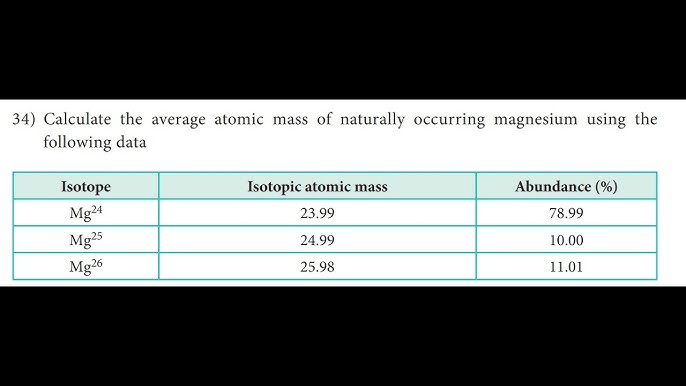
24.31 amu
Which element is in the same group as silicon, but also is an element Mendeleev boldly predicted the existence of before it was actually discovered? (Hint: It was discovered a European country)
Germanium
JJ Thomson used __________________ to discover the existence of tiny negatively charged subatomic particles called electrons.
cathode rays
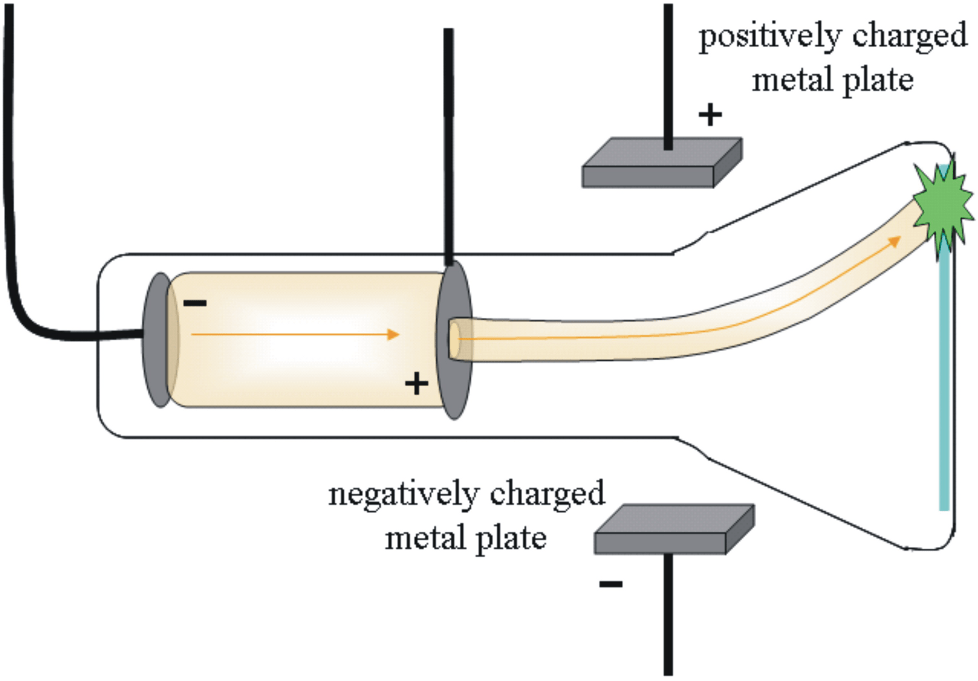
What is the name for elements that share properties of both metals and non-metals?
metalloids
What is the mass number of a period 4 halogen that has 45 neutrons?
80
What is the mass number of an isotope of chlorine that is 75.77% abundant and the other isotope is chlorine-37, which is only 24.23% abundant? Hint, find the average atomic mass from the periodic table and do some fancy mental math.
35
What is the name of the collection of families of elements, which exclude transition metals, the lanthanide series and the actinide series?
Main Group Elements (Group 1,2,13-18)