What is the simplest sum of whole number coeffecients for the reaction with magensium and oxygen?
What is five?
Which of the following when dissolved in water would produce an acidic substance?
1. MgO
2. Al2O3
3. SO2
What is SO2
Draw a endothermic reaction diagram
Give an example of a ampiprotic substance
Water, or HSO4- are some common examples
What are the functional groups present in this molecule?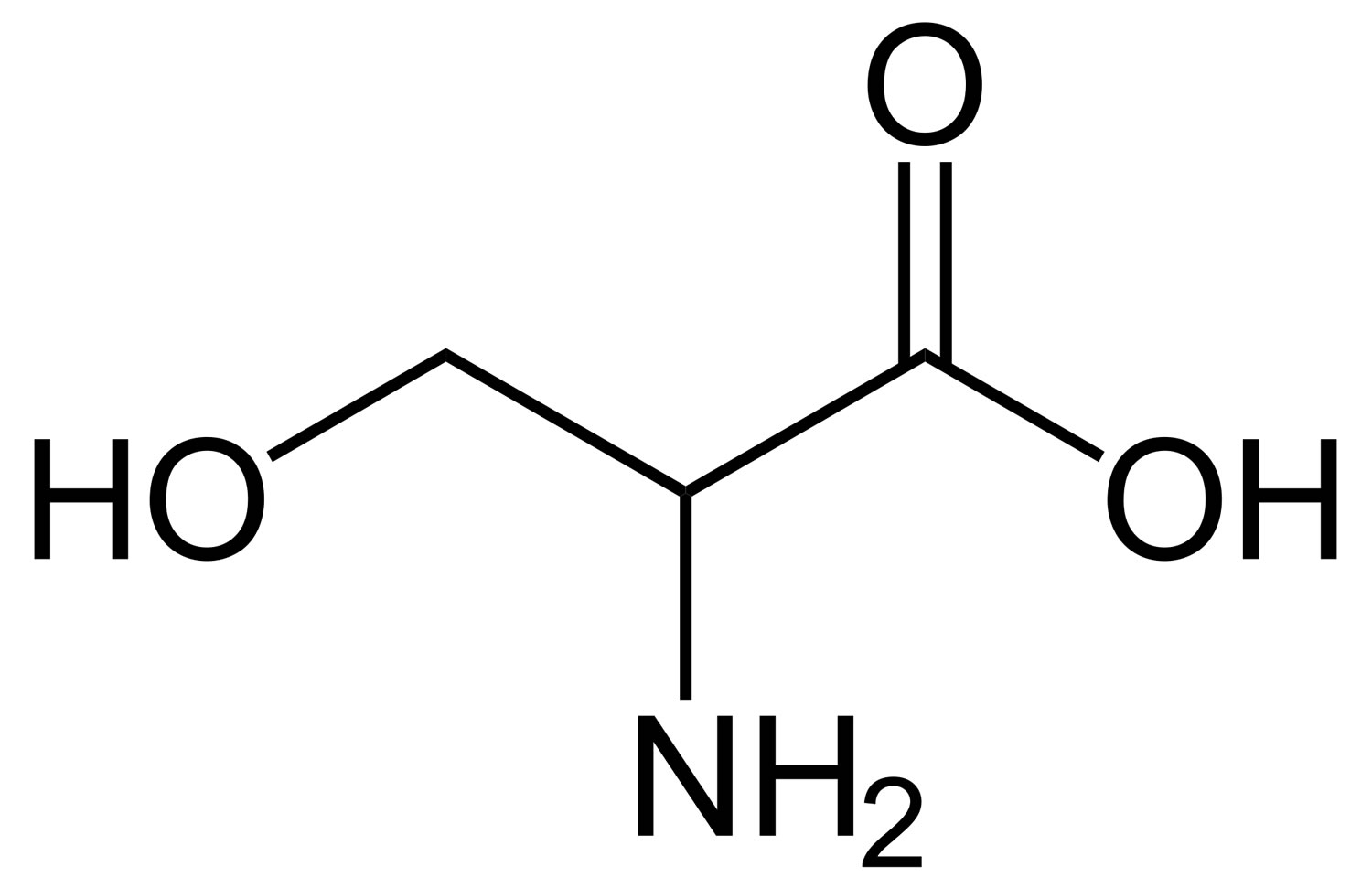
What is hydroxyl, carboxyl and amine
I have 47g of Ammonia at STP. How many dm3 of space will this occupy?
63dm3 (2 sif fig)
What is a metal?
An experiment was set up to measure the molar enthalpy of combustion for an alkane. Describe 3 potential source of error and assumptions made in the calculation?
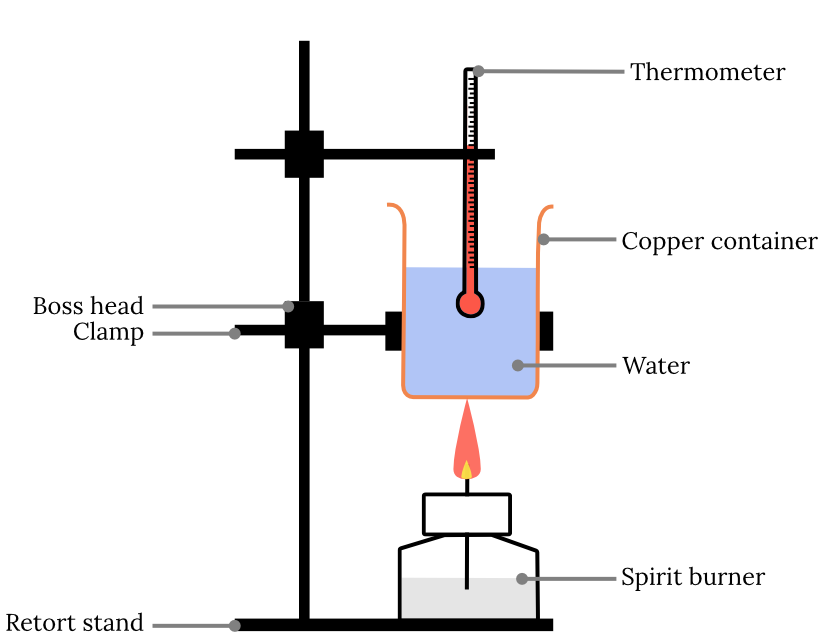
1. Water has a specific heat capacity of 4.18.
2. The specific heat capacity of the calorimeter does not affect the calculation.
3. Compustion is complete
4. The thermometer does not affect the specific heat capacity of the water (and is not touching the calorimeter)
5. No heat is lost between the calorimeter and the spirit burner.
6. All heat is retained in the calorimeter
Give an example of a weak and a strong base
Ammonia is a weak base (NH3), Strong bases contain alkali metals and hydroxides
Draw the structural formula of the next chemical in the homologous series after propan-1-ol

What is the capacity of a S and a P orbital?
What is 2 and 6
How many protons neutrons and electrons are in the following

Protons= 4
Neutrons= 5
Electrons = 2
A calorimetry experiment was performed with a data logger and temperature probe.
Calculate the delta T and explain why this is more accurate way of recording the temperature change?

What is 39- 24= 15
The calibration allows to measure the heat lost by the calorimeter and accomodate for this in our calculation of delta T.
Finish the statement. A change in a pH of 1 to 3 will result in a Hydrogen ion concentration that is ..... times weaker
What is 100
No oxidation can occur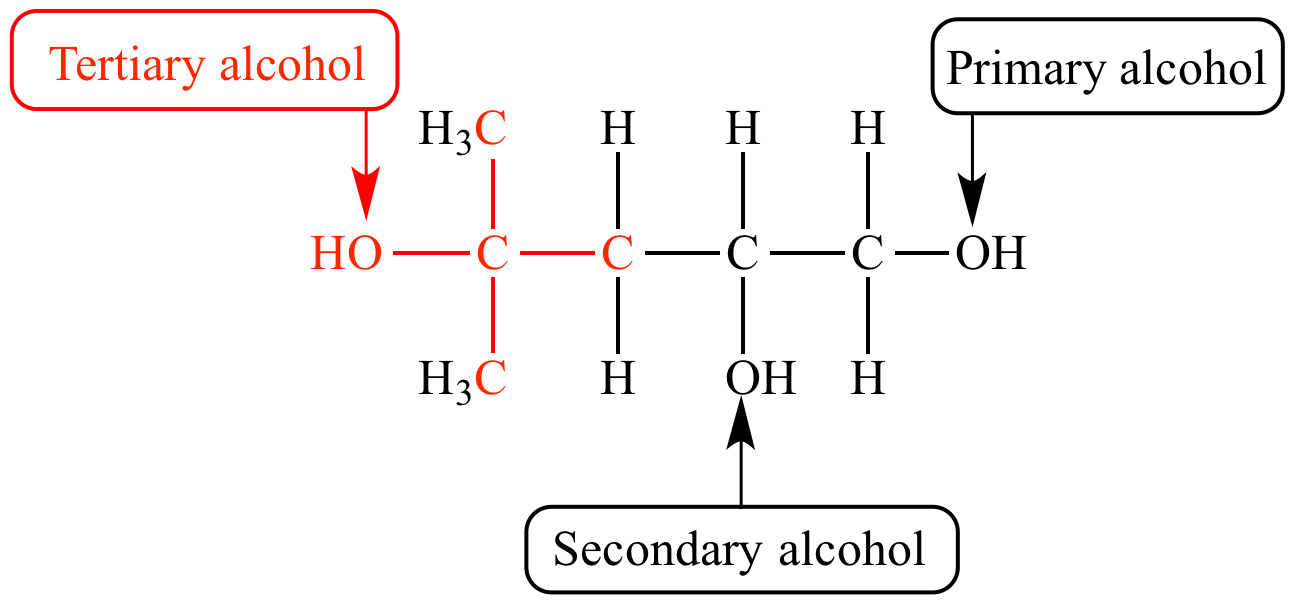
If I have 75.5% Cl- 35 and 24.5% Cl-37. What is the relative molecular mass? Answer to 2dp
What is 35.49
Which substance is most polar?
CH3Cl
CH4
CH3F
What is CH3F
An experiment took place reacting a primary alchohol (100% concentration) with potassium permanganate. The amount of primary alcohol was measured over time.
a) Identify the two possible products
b) Draw a second curve if the reaction was repeated with a (50% water and alcohol mixture)
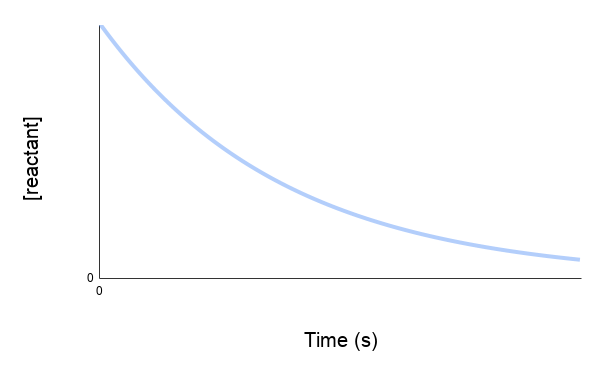
What is
a) An aldehyde and a carboxylic acid
b) The curve will only go down half as far and the reaction will be slower initially
Magnesium reacts with hydrchloric acid to form 2 products. Write a balanced chemical equation and identify which species undergoes oxidation and reduction in the equation.
Magnesium undergos oxidation, hydrogen undergoes reduction.
Chloromethane reacts with sodium hydroxide. Name the possible products.
Methanol and sodium chloride
What are 3 possible systematic errors when finding the empirical formula of magnesium oxide? 
Not all magnesium is converted to magnesium oxide
Some magnesium oxide mass is lost
Some magnesium is converted to magnesium nitride
Draw a model of a
a) Ionic substance
b) Metallic substance
Compare their structures in terms of free electrons

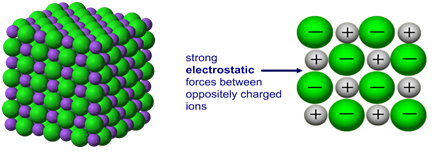 Electrons are free in metallic bonding. Electrons are free in an ionic substance- but only when it is molten
Electrons are free in metallic bonding. Electrons are free in an ionic substance- but only when it is molten
Identify the 3 requirements for a successful collision and 3 possible ways a reaction can be measured (eg. change in mass over time)
What is
a) Particles must collide, particles must collide with correct orientation, particles must collide with sufficient energy.
b) Change is color over time, change in conductivity over time, change in pH over time, change in temperature over time, change in volume of gas over time
A reaction took place as seen below:

a) Write half equations for the reaction.
b) The experiment was repeated changing zinc for another metal. However no reaction took place. Suggest an identify of the new metal.
a)
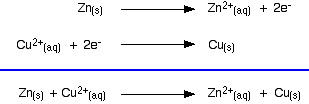
b) Any metal with a lower reactivity than copper (eg. Silver)
Calculate the IHD of morphine (C₁₇H₁₉NO₃)

What is 9?
