The number of protons in an atom
Atomic Number
What did Rutherford suggest about the structure of the atom based on the results of his gold foil experiment?
Positively charged mass in the center of the atom (he called it the nucleus),
Positively charged
Protons
Which group of the periodic table is the most reactive?
Group 1A, alkali metals
Although there are many illustrations and models fo atoms, have scientists actually seen atoms themselves?
NO
The sum of the protons and neutrons in the nucleus of an atom
Mass number
How many electrons does an atom of sodium have? Its atomic number is 11
11
Negatively charged
electrons
Why do elements in group (column) of the periodic table have similar properties?
they have the same number of valence electrons
All mater is made of particles called ________________, and two or more of these from different elements can join together to form_________ in chemical reactions.
atoms, compounds
Electrons that are in the highest energy level (the energy level farthest from the nucleus) of an atom of an element
Valence electrons
How many electrons are in in the first energy level?
2
Neutral Charge
neutrons
Which group is the least reactive?
Nobel gases
Where is the greatest part of the mass of an atom found
Nucleus
Matter cannot be created nor destroyed, but changes from one form into another
Law of conservation of mass
For an electron to move to a lower energy level, it has to ______________________ energy
lose
located in the nucleus
Protons and electrons
Who first organized the periodic table?
Dmitri Mendeleev
The atomic number for oxygen is 8. How many electrons does an atom of oxygen have?
8
Forms of the same element that contain equal numbers of protons but different numbers of neutrons in their nuclei, and hence differ in relative atomic mass but not in chemical properties
Isotopes
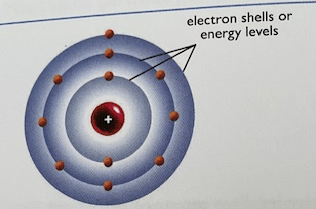
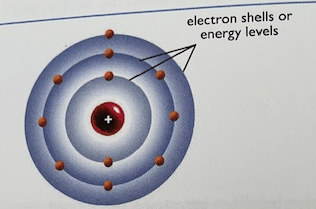
What is the name for this atomic model
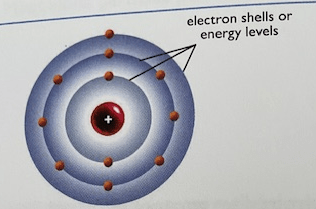
Bohr Model
The smallest of the three subatomic particles
electrons
How is the modern periodic table organized?
As you move from left to right across a period, the atomic number (protons) increases by 1
An atom of an element has a mass number of 38. It has 20 neutrons in its nucleus. How many protons does the atom have?
18