Name ONE of the methods of energy application that cause decomposition reactions. Giving the methods name gets DOUBLE points.
Electricity (Electrolysis)
Light (Photolysis)
Which of the following metals is the most reactive?
Mg, Fe, Li, Cu, Au
Double points for putting them in correct reactivity order (Most to least)
Li (Lithium)
Li>Mg>Fe>Cu>Au
Reaction rate is calculated by what equation?
(Delta Concentration)/(Delta time)
Nitrogen
What are the substances that can form between reactants and products?
Intermediates
Based on the below potentials, does the addition of metal X react in a solution containing Y+?
X+ + e- --> X = -1.4V
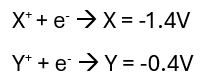
Yes.
The higher voltage will be the reduction reaction (reduction of Y+). This means the X metal will be oxidised and its voltage flipped to +1.4V. Since oxidation is the metal dissolving into solution, this will occur spontaneously due to the positive voltage of the reactions +1.4V + (-0.4V) = +1V
Decrease.
As concentration increase so too does reaction rate and vice versa.
What is the process where a solid changes directly into a gas?
Sublimination
What compounds are formed that signify complete combustion.
Carbon dioxide (CO2) and water (H2O)
Identify the anode and cathode if the reaction below occurred in a galvanic cell.
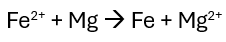
Anode = Mg (Magnesium)
Cathode = Fe (Iron)
RedCat and An Ox dictates that reduction occurs at cathode and oxidation at anode. Since iron is reducing, it will be the cathode.
According to collision theory, reactants must do what in order to react?
1. Collide
2. Have enough kinetic energy
3. Have correct orientation when colliding
Chlorine
Write the net ionic equation for the reaction between calcium chloride and potassium sulfate. Include all states
Ca2+(aq) + SO42-(aq) -> CaSO4(s)
Note: Using NAGSAG, sulfates have the exception CataStrophic Bat, making calcium sulfate insoluble.
The electrode's substance was not known in a galvanic cell. The voltage of the cell was measured to be 1.63V and the standard potential for the other electrode was found to be -1.3V. What was the standard potential of the unknown electrode?
-2.93V
Since the electrode is unknown, you use the formula Etotal = EA - EB
1.63V = -1.3V - EB
EB = -2.93V
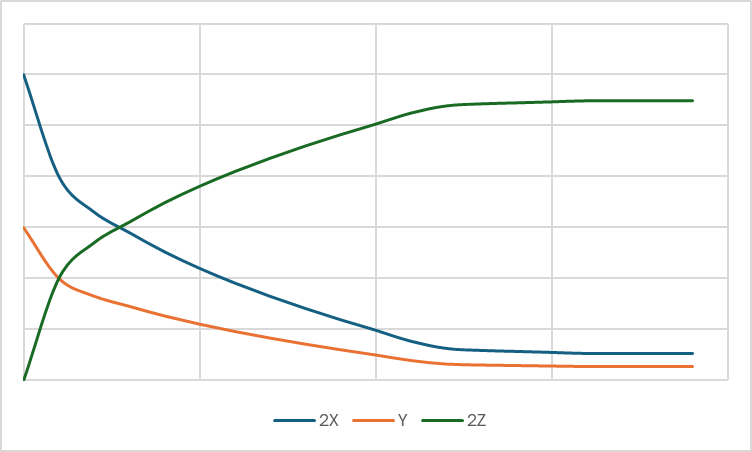
Draw a rate of reaction graph that has the concentration over time for a reaction with the following molar coefficients.
Note: Numerical values not required but does need to be to scale to some degree.
What principle explains the inability to know the position and the momentum of an electron at the same time?
Heisenberg's Uncertainty Principle
A reaction between solid lead carbonate and hydrochloric acid was conducted. Name both reaction types that occur in this reaction AND all products formed.
Reaction types: Acid-carbonate and precipitation
Products formed: CO2, H2O, PbCl2
A galvanic cell was constructed and produce a positive voltage. What would happen to the voltage of galvanic cell if it was constructed the same, but the anode solution concentration was decreased. Give a valid reason
Increased voltage
1. Reduced cations near the anode allow the new cations to move into the solution easier/faster.
Galvanic cells always use cations at the cathode and form cations at the anode. This means that increasing conc. at cathode and/or decreasing concentration at the anode will increase the voltage.
Two identical reactions were conducted where a factor was changed. The results are below.
Reaction 1: A 10% solution resulted in a 100mL solution with 5g of reactants after 35s
Reaction 2: 12g of products formed in a 50mL in just under 3 minutes
Was the factor that was changed one that causes a change in reaction rate?
Impossible to tell
The reactions have the same reaction based on change in concentration over change in time (approx. 0.14%/s) using the values provided. However,
1. You cannot use the concentration of reactants in one formula and products in another if they have different molar coefficients. E.g. if ratio is 2:1, then the 2 would change concentration twice as fast as the 1.
2. Grams also aren't comparable between different substances and reactants and products have to be different substances.
Draw the organic compound, benzene
