Mass
What is the amount of matter packed into a space?
When two molecules are bonded because they share electrons
What is a covalent bond?
A substance made up of all one kind of atom
What is an element?
A change in which the atoms or molecules in a substance stay the same
What is a Physical Change?
What is a proton?
This number is neutral on the pH scale
What is 7?
Matter
What is anything that takes up space and has mass?
These electrons located in the outer shell are responsible for bonding
What are valence electrons?
Molecule
What are two or more atoms of the same or different kinds bonded together?
A change that affects the type of molecules or atoms in a substance
What is a Chemical Change?
The negatively charge subatomic particle
What is an electron?
These substances taste sour and conduct electricity
What are acids?
The point at which a liquid becomes a gas by adding heat
What is the boiling point?
Positively charged ion
What is a cation?
Where you would look to find the number of protons in an atom, also called the atomic number
What is the number above the letter on the periodic table?
Combustibility
What is the ability to burn or explode?
These are the two subatomic particles that are found inside the nucleus of an atom
What are protons and neutrons?
These substances are slippery and taste bitter
What are bases?
The point at which a sold turns liquid by adding heat
What is melting point?
Negatively charged ion
What is an anion?
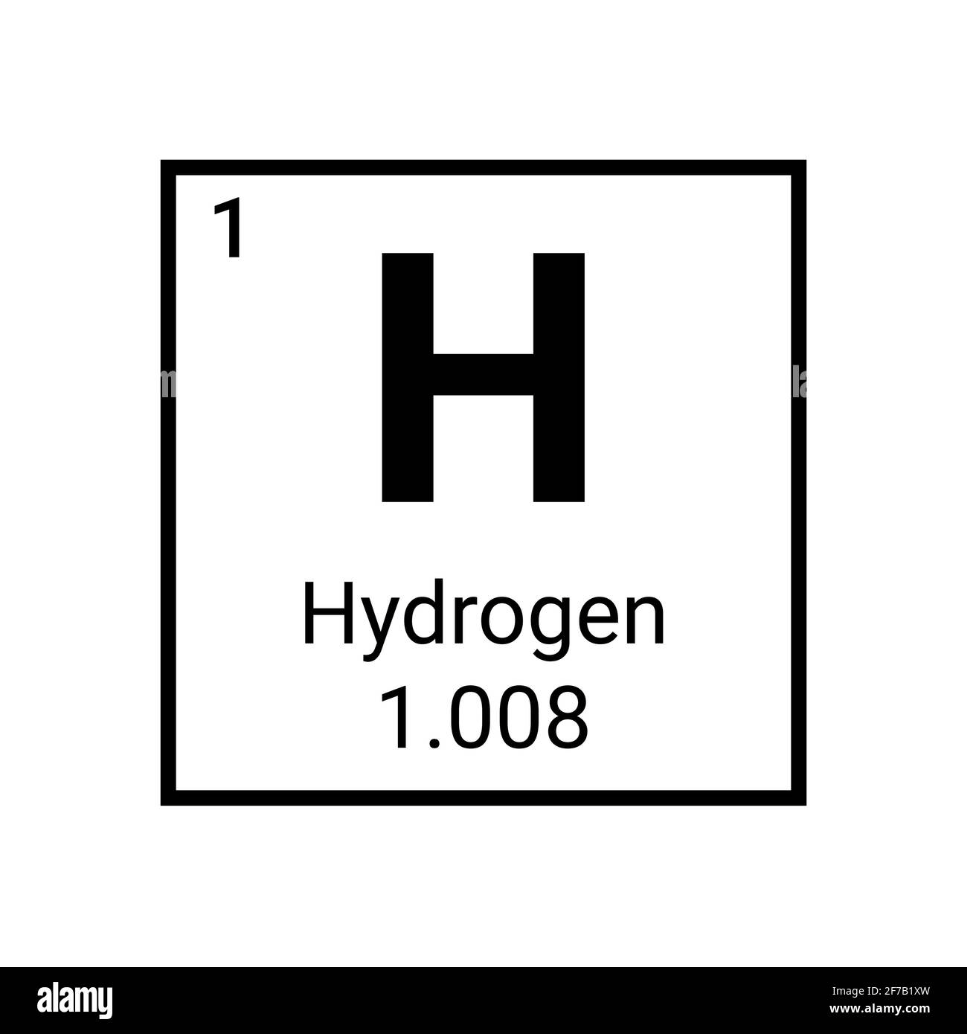
How many ELECTRONS does this element have?
What is 1?
An element or compound which has geometric sides as a solid
What is a crystal?
This happens when electrons gain energy
What is jumping to an outer orbit/energy level?
This is an example of a natural polymer
What is rubber, carbohydrate, sugars, DNA, protein?
The temperature at which a gas becomes a liquid
What is a condensation point?
This bond is created by positive an negative charges
What is an ionic bond?
The periodic table is arranged in this order
What is by atomic number/by size?
Rusting iron is an example of this kind of change
What is chemical?
This is how we find the number of neutrons in an atom
What is....it's equal to the number of protons?
Monomer
What is a small molecule made up of a single part?
What is happening (in terms of heat) when water vapor turns to water and then ice.
What is heat being subtracted?
These elements are located on the right side of the periodic table are very stable because they have all their electron shells filled
What are noble gasses?
A substance that contains different compounds and/or elements
What is a mixture?
An example of a homonuclear diatomic
What are nitrogen, oxygen, chlorine, hydrogen, etc?
What is strong force?
What is 0-6.9?