This substance that is used as a household cleaner has a strong odor and has a trigonal pyramid geometry...
What is ammonia.
The chemical CaF2 is named this...
What is diphosphorous tetraiodid
This substance is used in our Bunsen burners and has four hydrogen atoms attached to a central atom. (Name the molecular formula and geometry.)
What is CH4 (methane), tetrahedral
A nonpolar bond will form between two atoms of __________ electronegativity.
What is similar / identical electronegativities.
Rule that states that atoms gain, lose, or share electrons in order to gain stability and have the valence electrons of a noble gas?
What is Octet (or for Hydrogen/Neon, Duet) rule
Name the trend that is responsible for the attraction strength of an atom in a molecule towards the shared electrons in a bond.
What is electronegativity?
Fe2O3
What is Iron (III) Oxide?
Atoms in this family on the periodic table typically have three bonds and one lone pair of electrons...
What is the nitrogen family?
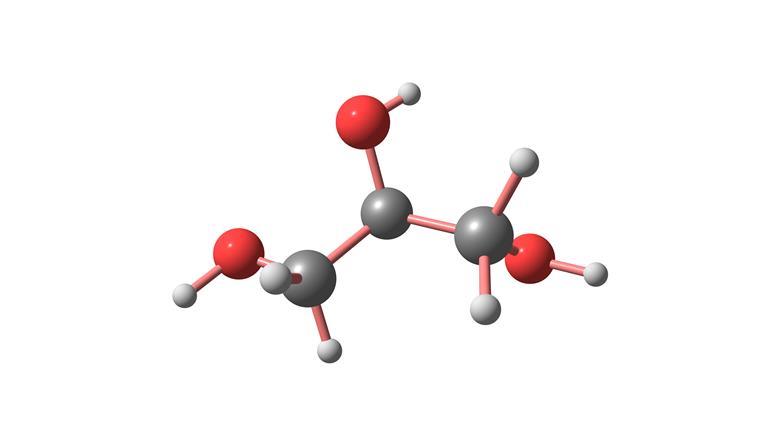
The reason why glycerol has such a high viscosity.
What is three hydrogen atoms capable of hydrogen force bonds.