What is this lab material?

Erlenmeyer Flask
What is the molar mass of water?
18.015 g/mol
What is an isotope?
An atom with a different number of neutrons.
How do you convert from moles of Carbon to Atoms of Carbon. Be VERY specific.
Use the molar mass of Carbon, which is 12.011 g/mol
What does a mass spectrometry graph measure?
The % abundance of isotopes of an element.
What are the steps you must follow to find the molecular formula from % mass?
find the empirical formula (using the "rhyming steps") calculate the empirical formula mass divide the molar mass by the empirical formula mass multiply the empirical formula by this number
What is this element likely to be called?
Boron (B)

What is this lab material?
Evaporation Dish
How many molecules are in 35.4 mol CO2?
2.13x10^25 molecules
What are the steps used in order to determine the empirical formula of a molecule from the percent make up?
percent to mass, mass to mole, divide by small, multiply by whole
Find the molecular formula for a compound whose molar mass is 186.072 and empirical formula is CH2O3.
C3H6O9
Which element is this likely to be?
Zirconium (Zr)
Find the answer to this problem with the correct number of significant figures.
How many TOTAL moles of NaCl and H2O are present when 12.34 grams of NaCl are added to 3.9572 moles of H2O?
4.21 moles of NaCl + H2O
Why can't you convert directly from particles to grams(and vice versa)?
The mole is a representation of the number of particles in a sample that a scientist can actually work with. There is a high probability of error if you were to use the mass of a single particle.
What is the empirical formula for a compound containing 50.0% C, 5.5% H, and 44.4% O?
C3H4O2
What is the molecular formula of a compound containing 62.0% Cr and 38.0%O whose molar mass is 176.3874 g/mol?
Cr2O4
Which Element is this most likely to be?
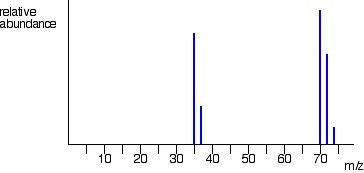
Chlorine (Cl/Cl2)