CH4
What is methane?
The skeletal structure of propane

Name me
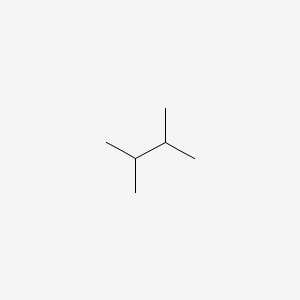
What is 2-methylhexane
Name me CH3CH2OH
What is ethanol
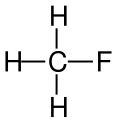
Draw the structural formula for fluoromethane
Alkane, Alkene or Alkyene

What is alkene?
The condensed formula for ethane
CH3CH3
Draw
2-ethylpentane
Name me (red is oxygen)

Propan-1-ol
Name me

Trichloromethane

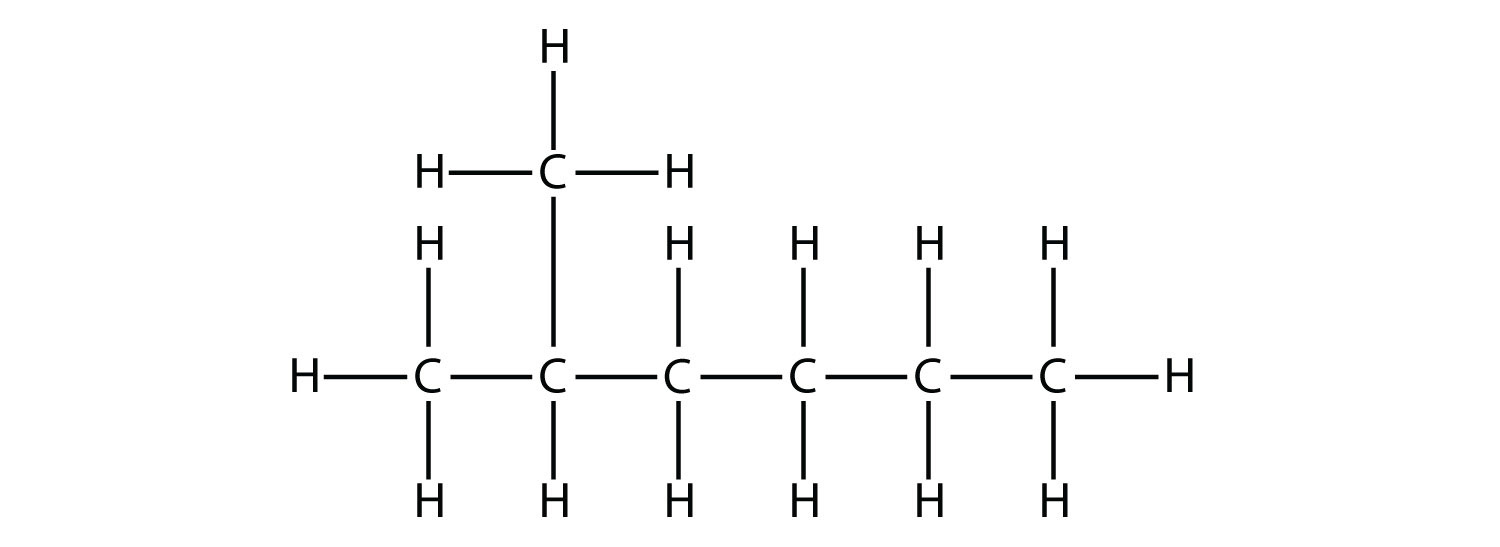
What is hexane?
The skeletal formula for propene

Explain why 4-methylpentane cannot exist. Suggest a better name.
What is 2-methylpentane?
This weak acid donates a hydrogen

What is ethanoic acid
1,1,2 trifluromethane
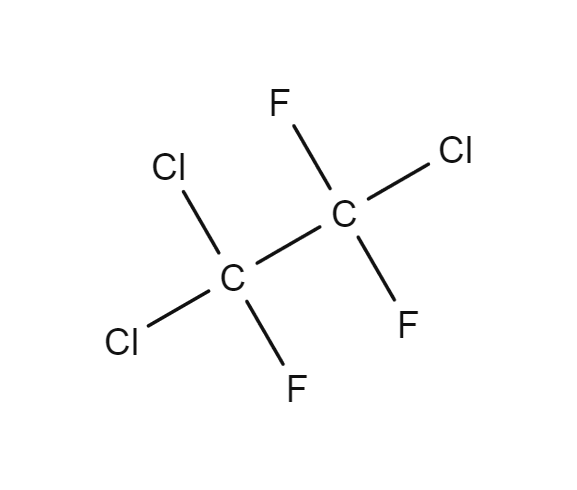
Which carbon carbon bond would be the shortest-
Alkane, Alkene, Alkyne
What is alkyne? (a triple bond)
Name me. CH3CHCHCH3
What is but-2-ene
Name me:

What is 3-ethyl-2,4-dimethylhexane
Write the condensed formula for propanoic acid
CH3CH2COOH
Name me

1,1,2-Trichloro-1,2,2-trifluorethane
What is the general formula (eg CnHx) for an alkane
What is CnH2n+2
What is 2,3 dimethyl butane
Isomers are chemicals with the same chemical formula but different structures.
How many isomers does butane have? Name it/them.
What is one? 2-methylpropane
Draw Pentan-2-ol?

Does this exist?
1-chloro, 2-iodo, 3,3 fluoropropane
Draw it
What is answers will vary