Add 273 Degrees to temperature
Kelvin
Metals
FCC,BCC, SC, HCP are types of
Crystal Structures

Bohr Model
Give the electron configuration of Sn4+ (Can use abbreviated form)
1 micron (um) is 10^x meters
-6
Polymers
Withstanding 800C degrees in thermal heat or a force of 10000N or corrosion resistance are examples of
Material Performance
Some say I'm small but I say Im noble. What do me and the rest of my group have in common
Full p orbital valence shells
The four electron quantum numbers are n, l, ml and ms. What does l represent?
Orbital shape
8.31 J/mol k
Gas Constant
Alumina is a high performing material found in high temperature applications.
Ceramics
Metals are now more commonly 3D printed using techniques like Laser Powder Bed Fusion or Binder Jet Printing. These are examples of
Processing
Electrically charged ions are formed by the transference of valence electrons from one atom type to another.
Ionic Bonding
Elastic modulus is an example of a material ________
Property
6.022x1023 molecules/mole
Avogadro's Number
Carbon Fiber is an example of this material hybrid
Composite
The yield strength of a material is 250 MPa
Property
1s2 2s2 2p6 3s2 3p6 3d8 4s2
Nickel
Isotropic
1.38x10-23 J/atom K
Boltzmann's Constant
Ionized gas grows bright neon colors
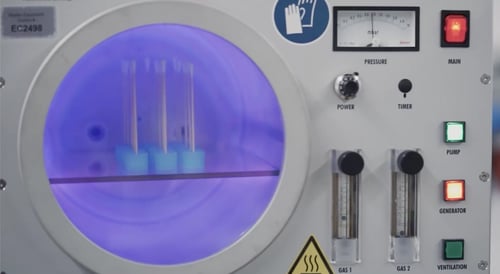
Plasma
High energy particles collide into densely packed atoms in a crystalline making it possible to capture them in photos called
Characterization/Microscopy
Compute the percent ionic character (%IC) of the interatomic bond that forms between carbon and hydrogen. (XC-2.5 XH-2.1)
3.9% Ionic, so mostly covalent (two non-metals)
Electrical conductivity in polymers is _______ than metals.
less than