8.1: Ionic Compounds
8.2: Molecular Compounds
8.A: Lewis Structures
8.4: Molar Mass
8.B: Naming Chemical Compounds
100
This is when something can be strong but still break easily.
What is brittle/ness?
100
This will tell you exactly how many atoms there are in an individual molecule.
What is a molecular formula?
100
When two polar molecules attract to each other you have an example of one of these.
What is a dipole-dipole attraction?
100
You can “float” a paperclip on water if you very carefully lay it flat on top of the water. This is due to what is known as _________.
What is surface tension?
100
This is the chemical formula for dinitrogen tetrafluoride.
What is N2F4?
200
If you have this, then you have a small molecule with a charge.
What is a polyatomic ion?
200
Molecules that are made from repeatedly bonding together smaller molecules are broadly called this.
What are polymers?
200
Draw the Lewis Dot Structure for dinitrogen monoxide.
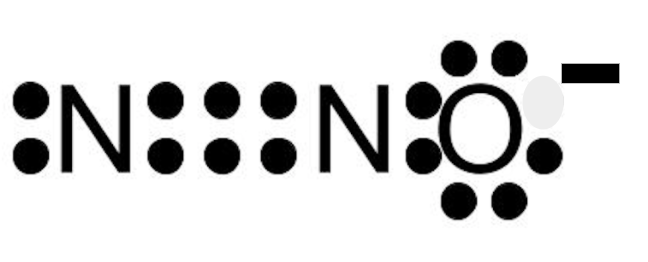
200
Calculate the molar mass for H2O.
What is 18.0148g/mole?
200
This is the chemical formula for phosphorous trichloride.
What is PCl3?
300
This is what you have when a charge moves in a directed way.
What is an electric current?
300
These are bonded together to form polymers.
What are monomers?
300
Draw the Lewis Dot Diagram for K2O.

300
Calculate the molar mass of NaNO3.
What is 84.994g/mole?
300
This is the name for the formula AgNO3.
What is silver nitrate?
400
What is one property shared by most ionic substances?
What is brittleness?
400
Polypropylene is only made from one monomer, which makes it one of these.
What is a homopolymer?
400
Draw the Lewis Dot Diagram for FeO.
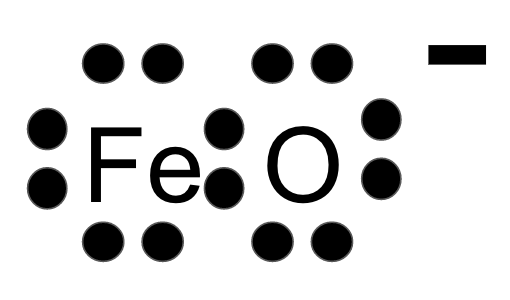
400
Calculate the molar mass of (NH4)2S?
What is 68.1422g/mole?
400
This is the chemical name for the formula CuSO4.
What is copper sulfate?