and Solubility
Solutions
The biggest percentage of a solution is made up of this, which is often a liquid.
What is a solvent?
This kind of solution is one that has water as its solvent.
What is aqueous?
When a substance will not dissolve it is said to be ____________.
What is insoluble?
There are several ways to calculate concentration. List three of them.
What are (any 3) grams per liter, percent mass , mass fraction, molality, mg/L, mg/mL, molarity, etc?
In a case where a salt dissolves and the solution absorbs heat, is the heat written on the products side of the arrow or the reactants side? Explain.
What is the reactants side? We do this to indicate that energy is absorbed, which means that the temperature of the solution decreases.
A molecule with a charge separation is called this.
What is polar?
Water that is condensed after boiling to separate solute particles from the water is called _____.
What is distilled water?
The amount of solute that will dissolve in a particular solvent is called its ___________ (at a given temperature and pressure).
What is solubility?
Describe how you would make a concentrated solution of lemonade.
What is: Increase the amount of lemon juice (the solute) used in making the lemonade?
Calculate the number of grams of potassium iodide (KI) needed to make one liter of a 1.0 M solution.
What is the molar mass of KI is 165.998 g/mole?
A substance is said to be _________ when it completely separates and disperses into the solution.
What is dissolved?
A solution that has very little solute dissolved in it is said to be ______.
What is dilute?
This is the energy released or absorbed when a solute dissolves in a solution.
What is heat of solution?
This is the molar mass of CuCl2.
What is 63.55 + 2(35.45) = 134.45 g/mole?
This is the number of moles in 1.75 g of solid Zn.
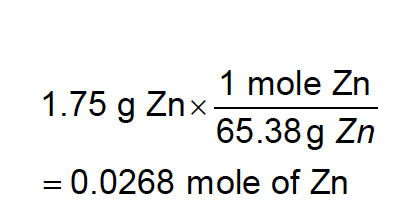 ?
?This is the substance dissolved in the solvent.
What is the solute?
If a solution is called _________ then it has a large amount of dissolved solute.
What is concentrated?
This is a solute capable of conducting electricity when it is dissolved.
What is an electrolyte?
When a chemical change takes place in a solution the water molecules either absorb heat or emit heat to the chemical solute. Name and describe these two processes.
It is called exothermic when heat is given off and endothermic when heat is absorbed.
What is the molarity of a solution that contains 8.5 g of NaCl in 250 mL of water?
What is the molar mass of NaCl is 58.55 g/mole 8.5 grams of NaCl corresponds to 8.5 g / 58.55 moles = 0.145 mole ? And the molarity is 0.145 mole / 0.25 L = 0.58 M
This property describes when intermolecular forces pull a liquid surface into the smallest possible area.
What is surface tension?
When the concentration of a solution is measured as moles of solute divided by liters of solution the concentration unit is this.
What is molarity?
Draw a picture of a water molecule. Label the positive ends and the negative ends of the molecule.
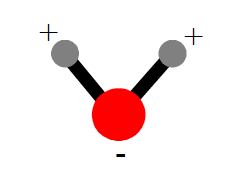 ?
?Describe why styrofoam coffee cups are good to use when we are investigating chemical changes in solution.
What is "because they insulate the solution from the environment; the solution does not lose or gain heat from the environment?"
Describe what entropy means, and what it means to decrease and increase it.
Entropy is a measure of thermodynamic randomness in a substance.
Decreasing the entropy means that molecules move less randomly.
Increasing the entropy means that molecules move more randomly.