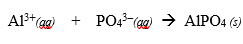It is also known as a precipitate
What is solid or (s)
(g)
What is a gas
NaCl (aq)
What is Na+ (aq) + Cl- (aq)
The liquid most aqueous solutions are dissolved into.
What is water
NaCl, NaOH, Zn(OH)2
Which are insoluble if any? (It doesn't belong with the others... if one exists.)
What is Zn(OH)2 - all group 1 metal-compounds are soluble in water.
Hydroxide is rarely soluble in water.
Balance the following molecular equation:
(15 seconds... 14, 13, 12)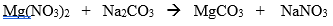
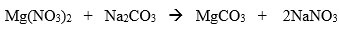
It is what does the dissolving.
What is a solvent
A whole number in front of an element or molecule
What is a coefficient
H2O (l)
What is H2O (l) It doesn't generally break into ions...
But if you said H+ and OH- that would be OK
True or False. H2O (l) is an aqueous solution
What is False It's a liquid - not ions
solute, solvent, solution, liquid
What is liquid
Give the complete ionic equation for the following already balanced equation:
35 seconds... 34, 33
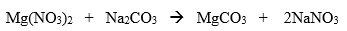
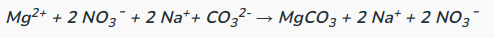
(Order on each side matters not.)
A gas or solid that dissolves into a liquid
What is solute
What does the "2" in H2O stand for?
What is a subscript telling you how many H atoms in the molecule
NH4OH (aq)
What is NH4+ (aq) + OH- (aq)
T - F Pb compounds NEVER are soluble in water?
What is false. NO3 (nitrates) are ALWAYS soluble, so Pb(NO3)2 - lead nitrate - is soluble in water.
aq, ->, g, l, s,
What is ->
Give the net ionic equation for the following complete ionic equation, showing the cancelled ions as well on your white board:
(40 seconds... 39, 38)
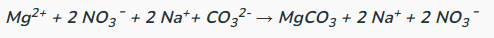
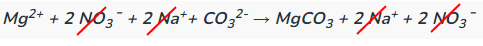
So:
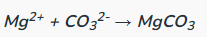
The ions that do not react in a chemical reaction
What are spectator ions
(aq)
What is aqueous
H2SO4 (aq)
What is 2H+ (aq) + SO42- (aq)
aqueous solution breaks down into these
What are its ions
The order of simplifying a chemical equation
What is balancing, total ionic equation, and net ionic equation
Determine the complete and net ionic equations for the following balanced equation, showing t he spectator ions as crossed out on each side, and (aq) or (s) where appropriate in the net equation:
(90 seconds... 89, 88, 65, 33...)
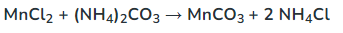
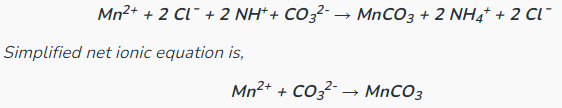
Better Net:
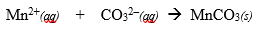
It better represents a chemical equation by specifically showing what reacted and what was produced.
What is a net ionic equation
(l)
What is the a liquid? ("aqueous" is not acceptable.)
Mg(OH)2 (aq)
What is Mg2+ (aq) + 2OH- (aq)
If all of the reactant and product ions are aqueous, what kind of reaction just took place?
What is none.
UGA, Georgia Tech, USC, Ohio State, Clemson
What is Georgia Tech - football (full points)
What is also USC - this year (1/2 points)
Determine the complete and net ionic equations for the following balanced equation, showing the spectator ions as crossed out on each side, and (aq) or (s) where appropriate in the net equation:
(90 seconds... 89, 88, 89, 88...)
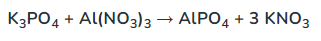
Complete:

Net: