An object whose density is 10 g/mL has a volume of 500.0 mL. What is its mass?
5000.0 g
The phase change from a solid to a gas is called _______________.
Sublimation
Define nuclear fission.
Nuclear fission is the splitting of a large nucleus into smaller ones, releasing energy.
This cold-weather sport uses a broom during play
Curling

You have been warm in your house all day, then step outside into a snowstorm to get the mail. Which statement is true?:
a. Cold moves from the environment into your body.
b. Heat moves from your body into the environment.
c. Both a. and b. happen.
b. Heat moves from your body into the environment.
Heat is energy, and energy is what moves. COLD is a LACK of energy, so it can't "move" because... well, nothing is there.
FREEBIE!!!!!
Yay!
The reason natural grass is cooler in the summer is because it has a _______ heat capacity versus an artificial turf field which has a _______ heat capacity.
High / Low
Draw a Bohr-Rutherford model of Chlorine (Cl)
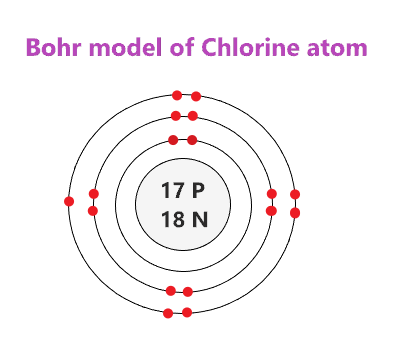
Of the elements that have 2 valence electrons, which element has the largest nucleus?
Radium (Ra)
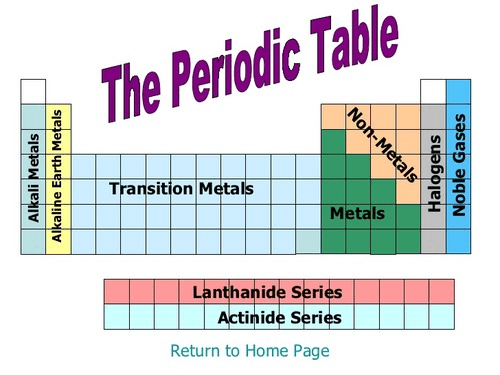
According to this chart, what would you classify O (Oxygen)?
Non-Metal
What is the difference between volume and mass?
Volume is the amount of space matter takes up, while mass is the amount of matter in an object.
FREEBIE!!!!!!
Yay!
What is the smallest state in the U.S.?
Rhode Island
What is the largest state in the U.S.?
Alaska
In a lab, a student places a hot metal block into a cup of cool water. If the metal block loses 300 Joules (J) of energy as it cools, and 225 J heats the water, how many Joules are absorbed by the container?
300J - 225J = 75J
Who is the most decorated Olympic athlete of all time?
Michael Phelps
Eggman is the enemy of who?
Sonic the Hedgehog
What is the isotope symbol for promethium-147?
What instrument do we use to measure volume?
Graduated cylinder.
FREEBIE!!!!!!
Yay!
What is the formula for mass?
M=DV
Describe the particle motion and arrangement in a solid.
Particles vibrate in place and are packed very closely together. Solids have a definite shape and volume.
What is the isotope name for the most common form of helium?
Helium-4
The kingdom of Hyrule is the main land in this video game.
The Legend of Zelda

In a lab, a student places a hot metal block into a cup of cool water. The metal block loses 300 Joules (J) of energy as it cools, 225 J heats the water, and some Joules are absorbed by the container. Which part of the system gained the most energy?:
The container, the block, the air, or the water
The water