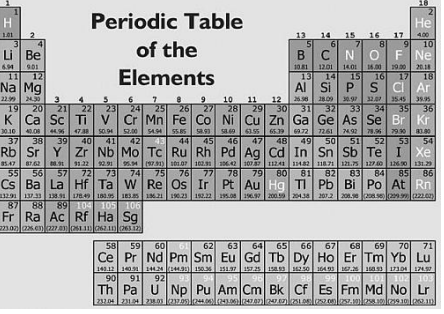
 The element sodium has the chemical symbol —
The element sodium has the chemical symbol —
A. S
B. Na
C. Sn
D. Sb
B. Na
Isotopes of an element have a different number of ___________________.
A. nuclei
B. protons
C. neutrons
D. electrons
C. neutrons
On the periodic table, calcium is in group 2. What is true about calcium?
A. it is a very reactive metal
B. it is a very reactive nonmetal
C. it is radioactive
D. it has four valence electrons
A. it is a very reactive metal
All elements in the same group have the same number of —
A. valence electrons
B. atomic mass units
C. neutrons
D. protons
A. valence electrons
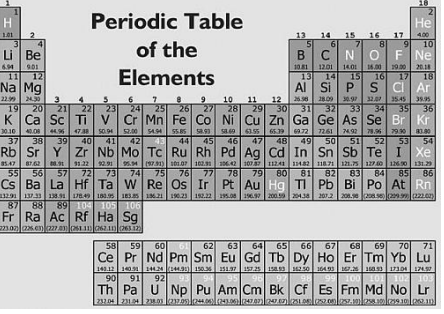 What is the chemical symbol of Hydrogen?
What is the chemical symbol of Hydrogen?
A. Hf
B. H
C. Hg
D. He
B. H
The electron has a —
A. negative charge
B. neutral charge
C. positive charge
D. static charge
A. negative charge
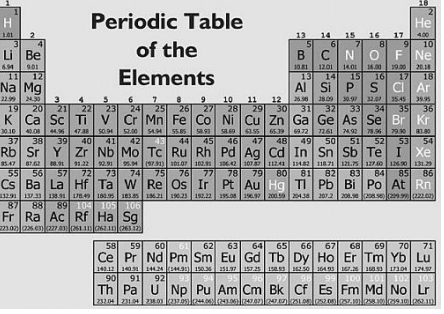 Referring to the periodic table, the element calcium should have the most properties in common with —
Referring to the periodic table, the element calcium should have the most properties in common with —
A. chlorine (Cl)
B. gold (Au)
C. magnesium (Mg)
D. potassium (K)
C. magnesium (Mg)
The atomic number of an element that has 6 protons and 6 neutrons is —
A. 6
B. 12
C. 3
D. 9
A. 6
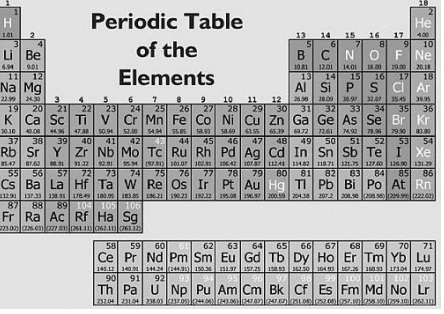 On the periodic table, the element sulfur is most like the element —
On the periodic table, the element sulfur is most like the element —
A. oxygen (O)
B. chlorine (Cl)
C. phosphorous (P)
D. calcium (Ca)
A. oxygen (O)
A student examines a periodic table.
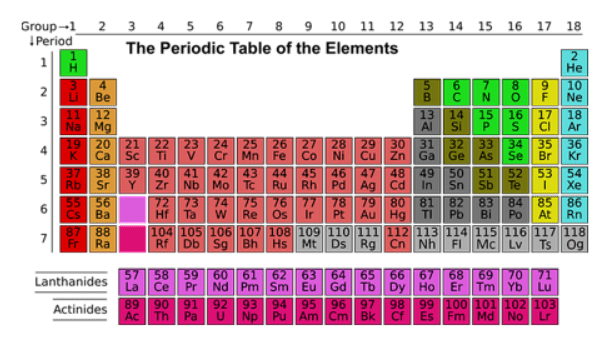
Which two inferences about sodium (Na) are true?
A. Sodium (Na) is a nonmetal, while aluminum (Al) is a metalloid.
B. Sodium (Na) has the least protons of any element in its period.
C. Sodium (Na) has 11 protons.
D. Sodium (Na) has a larger atomic number than magnesium (Mg).
B. Sodium (Na) has the least protons of any element in its period.
C. Sodium (Na) has 11 protons.
What distinguishes each element from all others, and gives it unique physical and chemical properties?
A. The number of protons
B. The element’s universal origin
C. The number of neutrons
D. The electron configuration
A. The number of protons
What type of charge does a neutron have?
A. negative
B. positive
C. neutral
D. static
C. neutral
A student receives a model of an atom that contains seven protons, neutrons, and electrons, and has an atomic mass of approximately 14. What procedure can the student use to identify the atom?
A. The student can reference the periodic table and find the element that has an atomic number of 7, which represents the number of neutrons in the atom.
B. The student can reference the periodic table and find the element that has an atomic number of 14, which represents the number of protons and neutrons in the atom.
C. The student can reference the periodic table and find the element that has an atomic number of 14, which represents the number of electrons and neutrons in the atom.
D. The student can reference the periodic table and find the element that has an atomic number of 7, which represents the number of protons in the atom.
D. The student can reference the periodic table and find the element that has an atomic number of 7, which represents the number of protons in the atom.
Which group of elements are shiny and are good conductors of both heat and electricity?
A. Radioactives
B. Metalloids
C. Metals
D. Nonmetals
C. Metals
Changing liquid water into steam is an example of — A. physical change
B. chemical change
C. identity change
D. bonding change
A. physical change
What is an example of a physical change in matter?
A. Iron rusting
B. Fire burning
C. Water freezing
D. Cake cooking
C. Water freezing
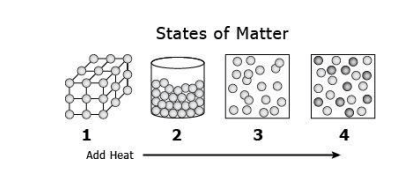 The matter seen in number 1 has what type of movement between atoms?
The matter seen in number 1 has what type of movement between atoms?
A. Liquid movement
B. Extremely small movement
C. No movement
D. Free movement
B. Extremely small movement
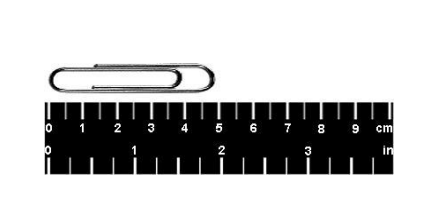 Kyle uses a meter stick to measure a paperclip. What length is the paperclip shown, in SI (metric) units?
Kyle uses a meter stick to measure a paperclip. What length is the paperclip shown, in SI (metric) units?
A. 5.0 m
B. 5.0 cm
C. 2 in
D. .005 cm
B. 5.0 cm
A measure of how much mass is contained in a given volume is called —
A. cubic measurement
B. density
C. volume
D. area
B. density
Helium is a gas at room temperature. This is an example of a —
A. compound
B. chemical property
C. physical property
D. mixture
C. physical property
Which part of the atom accounts for the majority of mass?
A. Number of neutrons
B. Electron cloud
C. Nucleus
D. Number of protons
C. Nucleus
Which subatomic particle has a positive charge and is found in the nucleus?
A. Neutron
B. Proton
C. Nucleus
D. Electron
B. Proton
Where is most of the mass of an atom located?
A. In the electron
B. Electron cloud
C. In the nucleus
D. Evenly spread throughout the atom
C. In the nucleus
 What conclusion can be drawn from the chart?
What conclusion can be drawn from the chart?
A. Yellowfin Tuna swim faster than Sailfish
B. Bluefin Tuna swim faster than Yellowfin Tuna.
C. Yellowfin Tuna swim faster than Bluefin Tuna.
D. All tuna fish in the ocean swim slowly.
C. Yellowfin Tuna swim faster than Bluefin Tuna.
Ice Cubes Melting in Lemonade On a sweltering hot summer day, two friends set up a lemonade stand in front of their house. They made the lemonade in the kitchen and take it outdoors to the stand. Their neighbor stops by, so one friend pours a cup of lemonade for them. The neighbor enjoys the refreshment, but says the lemonade would be even better if it was colder. The two friends have an idea that ice cubes could reduce the temperature of the lemonade. So, they dash into the house and bring a bowl of ice cubes out to the lemonade stand. They add the ice cubes to the pitcher of lemonade.
When do the water molecules in the ice cubes have their lowest particle motion?
A. After the ice cubes fully melted into the lemonade
B. Before Kim put the ice cubes into the pitcher of lemonade
C. After William drank the lemonade
D. While the ice cubes were melting in the lemonade
B. Before Kim put the ice cubes into the pitcher of lemonade
Sharayas wants to know if the amount of salt in an ice cube will affect the rate at which it thaws in room temperature water. What is the dependent variable in this experiment?
A. The amount of salt in each ice cube
B. The temperature of the water
C. The time it takes the ice cube to thaw
D. The time it took the ice cubes to freeze
C. The time it takes the ice cube to thaw
Beth is trying to break a record for the biggest bubble gum bubble. She usually chews Pop! bubble gum, but she will test different types of bubble gum to see which one will blow the biggest bubble. She compares the other brands to her standard, Pop! Beth uses Pop! bubble gum as her —
A. constant
B. control
C. dependent variable
D. independent variable
B. control
Kayla knows that a block of wood has a volume of 30cm 3. What tool would she need to use to calculate the density of the block?
A. a metric ruler
B. a balance
C. a graduated cylinder
D. a spring scale
B. a balance
 According to the diagram, what does the evidence show?
According to the diagram, what does the evidence show?
A. Cooler air has a higher relative humidity.
B. Warmer air has a higher relative humidity.
C. Air temperature is dependent on relative humidity.
D. Air temperature and relative humidity are unrelated.
A. Cooler air has a higher relative humidity.
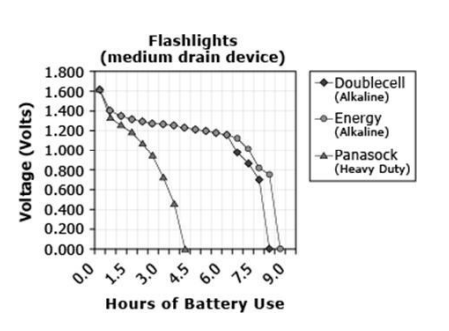 What is a correct statement about the Energy battery?
What is a correct statement about the Energy battery?
A. The Energy battery did not have any voltage after 4.5 hours of use.
B. The Energy battery had the shortest battery life of all batteries tested.
C. The Energy battery had a voltage of 0.700 after 8.9 hours of use.
D. The Energy battery had a voltage of 1.4 V at the beginning of the experiment.
C. The Energy battery had a voltage of 0.700 after 8.9 hours of use.