Give the formula for the following name: Dichlorine Heptaoxide
Cl2O7
Give the name for the following formula: N2O
Dinitrogen Monoxide
The two types of elements that make up an ionic compound
Metal and Nonmetal
The elements that always need roman numerals
Transition metals
Name this compound:
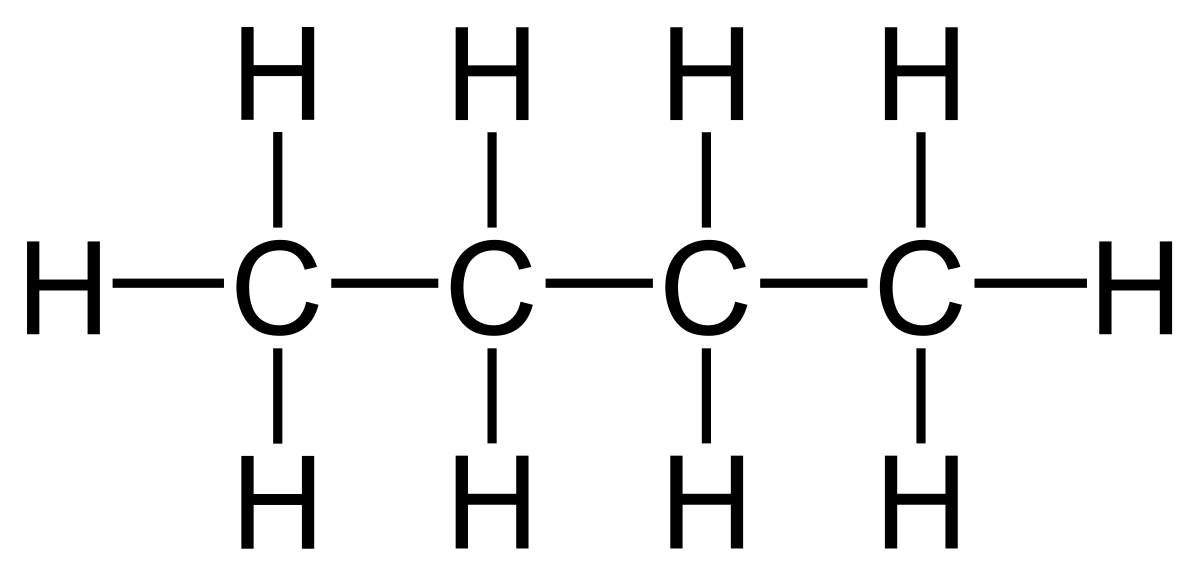
Butane
Give the Formula for the following name: Lithium Oxide
Li2O
Give the name for the following formula: HCl
Hydrochloric acid
The two types of compounds that make up a covalent compound.
Two nonmetal
The compounds that always need prefixes
Covalent Compounds
Name this compound:
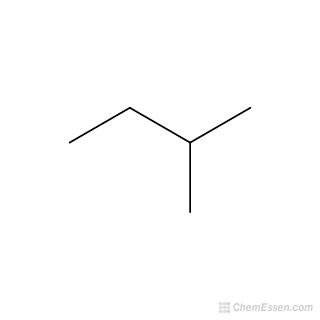
2-methylbutane
PbO2
Give the name for the following formula: NaNO3
Sodium Nitrate
The element that all acids contain
Hydrogen
The prefix used when there is NO polyatomic ion in the acid.
Hydro
Name all the functional groups in this blood pressure compound (Metoprolol):
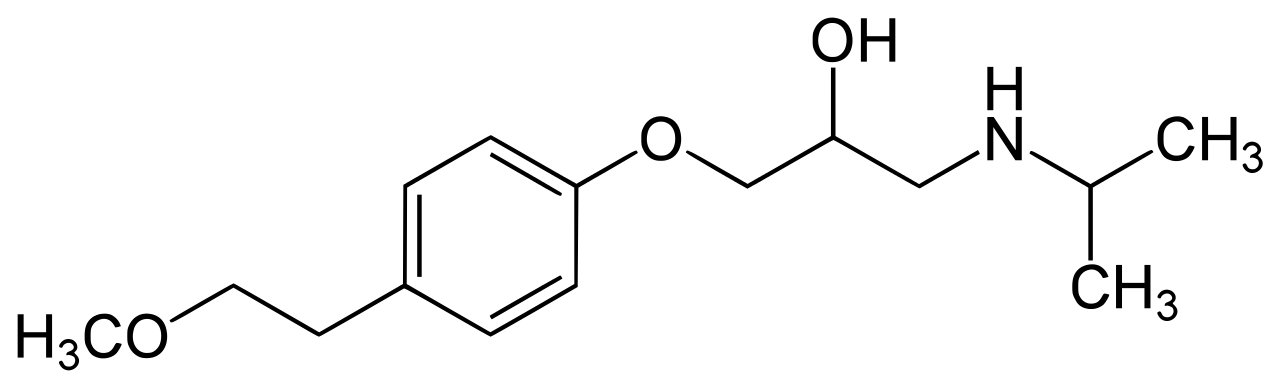
Amine, Alcholol, ether
Give the formula for the following name: phosphous acid
H3PO3
Give the name for the following formula: AgClO4
Silver Perchlorate
Organic compounds that contain at least one double bonded carbon
Alkenes
Ionic Compounds
Name this functional group:
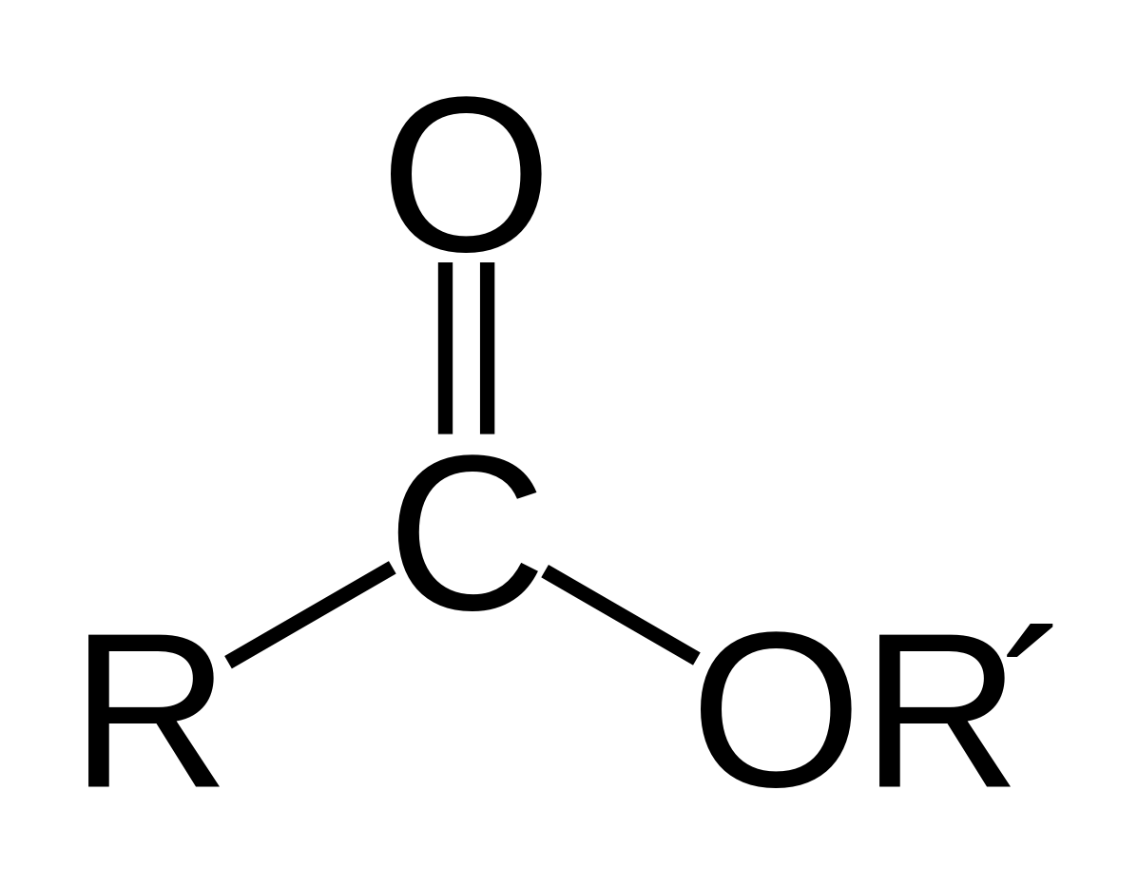
Ester
Give the formula for the following name: Ammonium Carbonate
(NH4)2CO3
Give the name for the following formula: NaIO2
Sodium Iodite
Molecular or Binary compounds.
The types of compounds that have low melting points.
Covalent compounds.
Name this compound: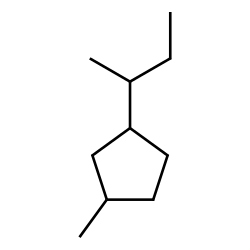
1-butyl-3-methylcyclopentane