How many neutrons does Carbon 14 have?
8
Which particle can only be stopped by lead or thick concrete?
Gamma
What type of radiation is this?
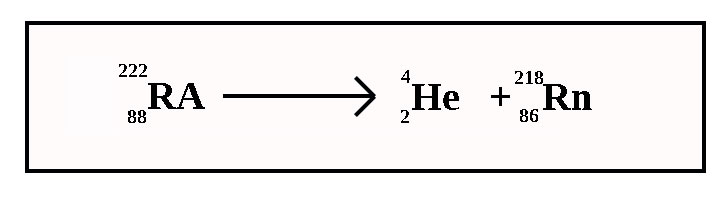
Alpha
What % of unstable isotopes are left after 2 half lives?
25%
This only happens naturally in the sun and stars.
Fusion
What does the top number represent in isotope notation?
atomic mass
What is the charge of a Beta particle?
-1
What is the missing number?
9943Tc -----> 00Y + ?43Tc
99
What is the half life of this isotope?
8 days
What type of decay breaks apart larger isotopes?
Fission
What is the mass of Carbon 12?
12
Which particle symbol is y00?
Gamma
What type of radiation is this?
Beta
12.5%
What is the most common use for nuclear fission?
Energy
Isotopes have the same number of protons but different amounts of?
neutrons
What is the mass of an Alpha particle?
4
What is the missing particle?
23892U ------ ??? + 23893U
Beta 0-1e
How much is left after 2 half lives?
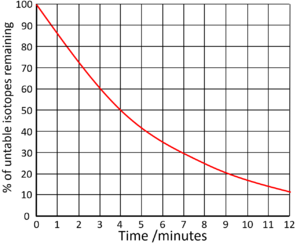
25%
What are the only two element isotopes that go through nuclear fission?
Uranium and Plutonium
How many neutrons does Oxygen 18 have?
10
Which two particles have 0 mass?
Gamma and Beta
What is the missing number?
23892U ------> 42He + ???90Th
234
What is the half life of this isotope?

6 days
Nuclear fission heats water to create steam that spins a turbine to generate electricity.