The splitting apart of breaking up of U-235 is defined as what?
Nuclear Fission
What are the 3 types of radiation that we talked about in class?
Daily Double: What are alternative names for each type of radiation?
Alpha, beta, gamma
Helium, Electron, High Energy Light
In chemical reactions, electrons are responsible for the new products. What subatomic particles are at play in nuclear reactions?
All of them. Protons, neutrons, and electrons.
What is one beneficial use of radiation?
The ratio of uranium-238 to lead-206 in a mineral is used to determine
A. age B. density C. solubility D. composition
Which element is used for dating archaeological discoveries? A. carbon-12 B. carbon-13 C. carbon-14 D. carbon-15
Radiation therapy, radiotracing, carbon dating
238U to 206 Pb is age and C-14 is archaeological discoveries
Daily Double: As a sample of a radioactive element decays, its half-life A. decreases B. increases C. remains the same
What is the total mass of a 10 gram sample of 42K that will remain unchanged after 12.4 hours? A. 2.5 g B. 5.0 g C. 7.5 g D. 10 g
C. Remains the same because half life is the time it takes for 50% of the radioactive isotope to decay therefore times does not change
B. 5 grams because only one half life occurred
We would divided the total time by the amount of time needed to achieve the number of half life.
12.4 hr /12.36 =1 half life
Two atoms that combine together and release a ton of energy in the process is defined as what?
Nuclear Fusion
List the types of decay in order of penetrating power (greatest to least).
Gamma, beta, alpha
11. Which equation represents nuclear disintegration resulting in release of a beta particle?
A. 220Fr + 4He --> 224Ac B. 239Pu --> 235U + 4He
C. 32P + 0e --> 32Si D. 198Au --> 198Hg + 0e
Explain your thinking: For Daily Double
What happens to the mass number in beta radiation? What happens to the atomic number in beta radiation?
D. 198Au --> 198Hg + 0e
Mass # stays the same. Atomic Number Goes up by one.
What device is used to detect radiation?
Double the points if you can say the units:
Hint : Video
A Geiger counter
Units: Bequerels, Curie, micro-sieverts /hr & Roentgens /hr
27. An 80 milligram sample of a radioactive isotope decays to 5 milligrams in 32 days. What is the half-life of this element?
A. 8 days B. 2 days C. 16 days D. 4 days
80--> 40 --> 20 --> 10 --> 5
4 half lives due to 4 arrows
Total time/time for 1 half life
4 half lives/ 1 half life = 32 days / x days
Cross multiply = 4*x = 32* 1--> x= 32/4= 8 days
Both Fission and Fusion reactions release massive amounts of what in addition to their respective atomic nuclei?
Energy
Which group of nuclear emissions is listed in order of increasing charge?
A. alpha particle, beta particle, gamma radiation
B. gamma radiation, alpha particle, beta particle
C. positron, alpha particle, neutron
D. neutron, positron, alpha particle
Daily Double: if you can draw the symbolic notation with the masses & respective charges for an electron, gamma, positron, proton, alpha particle:
D. neutron, positron, alpha particle (0, +1, +2)
0e (electrons) has charge of -1 , 0Y (gamma) has a charge of 0, 1n has a charge of 0, 0e (positron) has a charge of +1, 1P (Proton) has charge of +1, 4He (alpha particle) has charge of +2 ,
Which equation represents alpha decay?
A. 116In --> 116Sn + X B. 234Th --> 234Pa + X
C. 38K --> 38Ar + X D. 222Rn --> 218Po + X
Daily Double- Explain your thinking: Describe why in alpha radiation the mass number decreases by 4 and the atomic number decreases by 2
D. 222Rn --> 218Po + X
X= 4He
Daily Double:
2 protons + 2 neutrons = Change in mass of 4
2 protons = Change in atomic number of 2
Cobalt-60 and iodine-131 are radioactive isotopes that are used in ....
A. dating geologic formations B. industrial measurements
C. medical procedures D. nuclear power
Hint: These tracers are used in (daily double if you can tell me exactly what their used for)
C. Medical Procedures
Iodine used to treat hyperthyroidism & Cobalt-60 is used as a reactor in a medical device that fights rain tumors and blood vessel deformities
What is the number of hours required for potassium-42 to undergo 3 half-life periods? A. 6.2 hours B. 12.4 hours C. 24.8 hours D. 37.2 hours
1 Half-Life = 12.4 hours
2 Half-Life = 24.8 Hours
3 Half- Life = 37.2 Hours
What subatomic particle is primarily responsible for causing chain reactions involved in fission?
Hint: Bullet
Neutrons!
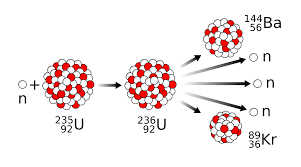
Transmutation is defined as...
Daily Double: Name the two types of transmutation
Which One is spontaneous?
The changing of a nucleus of one element into the nucleus of another element (by gaining or losing nucleons). This change always turns the unstable element into a more stable element.
There are two types of transmutation.
Natural vs Artificial Transmutation
12. Given the nuclear reaction:(Z) 60Co --> 0e+60Ni
Given the reaction: (Y) 27Al + 4He --> 30P + 1n
This reaction is an example of:
A. Fission B. fusion C. Artificial transmutation
D. Natural transmutation
(z) D. natural transmutation
(y) C. Artificial transmutation
Radioisotopes that have medical use as diagnostic injections have characteristics of A. long half-lives and quick elimination from the body B. short half-lives and quick elimination from the body C. long half-lives and slow elimination from the body D. short half-lives and slow elimination from the body
Brain tumors can be located by using an isotope of A. carbon-14 B. iodine-131 C. technetium-99 D. uranium-238
Radioisotopes that have medical use as diagnostic injections have characteristics of B. short half-lives and quick elimination from the body
Brain tumors can be located by using an isotope of C. Technetium-99
A radioactive isotope has a half-life of 10 years. What fraction of the original mass will remain unchanged after 50 years?
A. 1/2 B. 1/8 C. 1/16 D. 1/32
Total Time/Half-Life = # of Half Lives
50 years / 10 years =5 Half Life
(1/2)5 = 1/32
Name an advantage that fusion has over fission?
More energy is released, Fusion products are not radioactive, and or less dangerous no chain reaction (Any of these three)
Which equation represents a spontaneous nuclear decay? A. C + O2 --> CO2 B. H2CO3 --> CO2 + H2O C. 27Al + 4He --> 30P + 1n D. 90Sr --> 0e + 90Y
Explain your thinking: Natural and Artificial Transmutation difference
(Hint: How many reactants?)
D) 90Sr --> 0e + 90Y
Natural Transmutation Begins with one unstable nucleus that spontaneously decays. These reactions always have ONE REACTANT.
Artificial Transmutation is caused by bombarding a stable nucleus with high energy particles. These reactions always have TWO REACTANTS.
Daily Double: Part 1: Which fields are used in accelerators to speed up charged particles?
A. magnetic fields, only
B. electric fields, only
C. magnetic and electric fields
D. magnetic and gravitational fields
Part 2: In which list can all particles be accelerated by an electric field?
A. alpha particles, beta particles, and neutrons
B. alpha particles, beta particles, and protons
C. alpha particles, protons, and neutrons
D. beta particles, protons, and neutrons
C. magnetic and electric fields
B. alpha particles, beta particles, and protons
because they all have charge
What is a nucleon?
Explain why in beta radiation the mass number changes by 0 and the atomic number increases by 1
Subatomic Particles that reside in the nucleus such as protons and neutrons
Neutron gets changed into a proton and an electron. Proton mass = neutron mass so it doesn't change, but atomic number does change because protons increased.
Which of the following 10-grain samples of radioisotope will decay to the greatest extent and least in 28 days?
A. 32P B. 85Kr C. 220Fr D. 131I
You need reword this question it asking which element has the shortest half or most cycles since
220Fr = 27.4 seconds it would
32P = 14.28 days
B. 85Kr = 10.73 years
D. 131I =8.02 days